|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
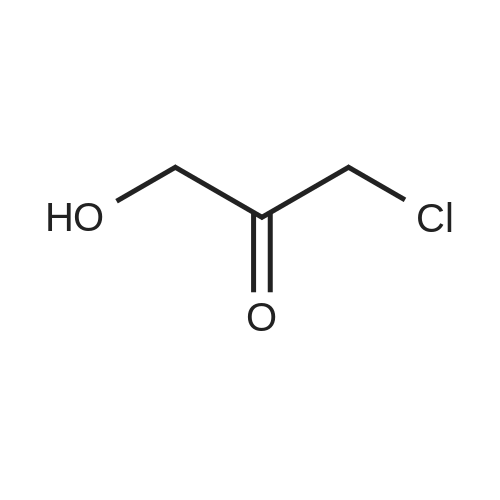
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With potassium dihydrogenphosphate; D-glucose; In water; at 30℃; for 24h;pH 5.5;Aqueous phophate buffer; Microbiological reaction; |
(Example 1) A liquid medium (pH 7.0) containing 4% of glucose, 0.3% of yeast extract, 1.3% of KH2PO4, 0.7% of (NH4)2HPO4, 0.01% of NaCl, 0.08% of MgSO4·7H2O, 0.006% of ZnSO4·7H2O, 0.009% of FeSO4·7H2O, 0.0005% of CuSO4·5H2O, and 0.001% of MnSO4·4-5H2O was prepared, and 5 ml of the liquid medium was injected into each of large test tubes and then sterilized with steam at 120C for 20 minutes. Then, one platinum loop of the microorganisms of each of the genera shown in Table 1 and 2 was inoculated into the liquid medium, and cultured with shaking at 30C for 2 to 3 days. Cells were collected from each of the culture broths by centrifugation, washed with water and then suspended in 1 ml of a 0.1M phosphate buffer (pH 5.5). Then, 0.5 ml of the cell suspension was mixed with 0.5 ml of a 0.1M KH2PO4 aqueous solution containing 5 mg of 1-chloro-3-hydroxyacetone and 20 mg of glucose, and the resultant mixture was placed in a test tube with a stopper and shaken at 30C for 24 hours. After completion of reaction, a saturated amount of ammonium sulfate was added to the reaction solution, and extraction was performed with an equivalent volume of ethyl acetate. |
|
With D-glucose; NAD; In water; at 30℃; for 2h;Microbiological reaction; |
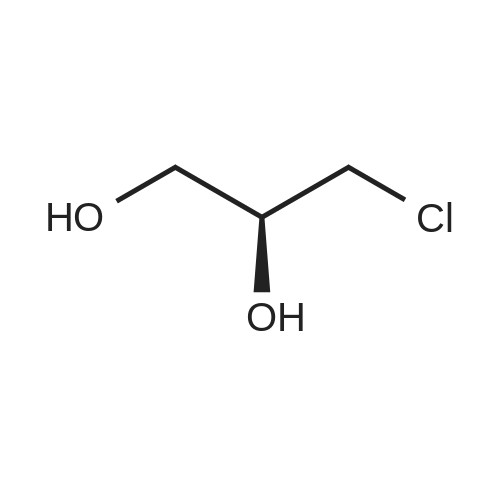
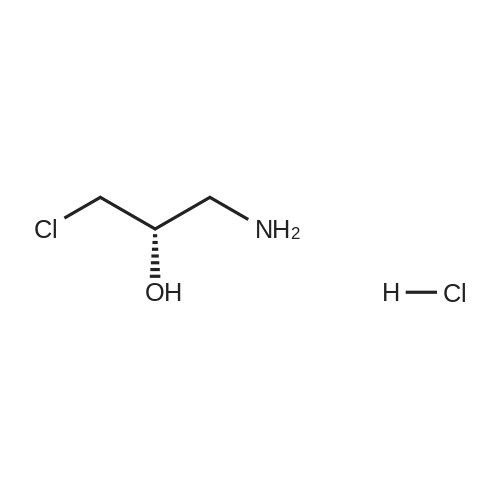
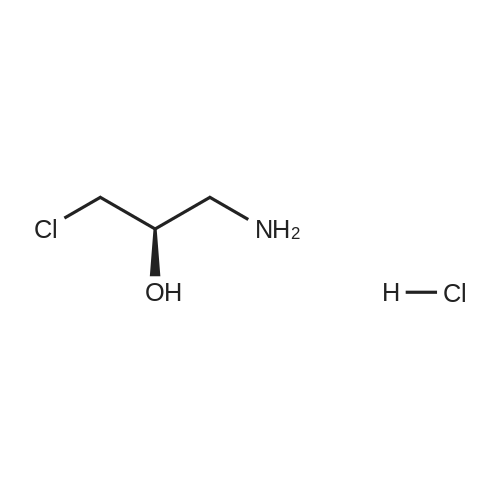
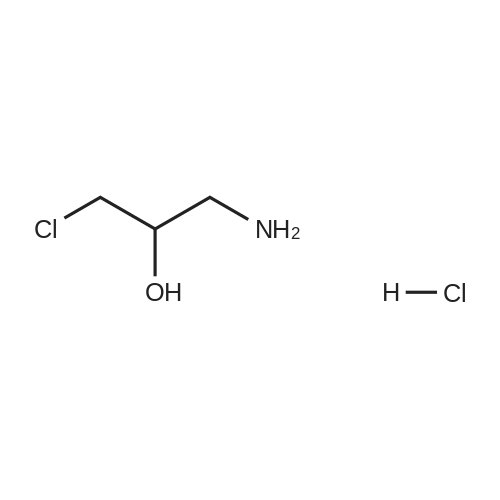
5(Example 13) Method for synthesizing (S)-3-chloro1,2-propanediol[0091] Recombinant Escherichia coli HB101 (pNTCRG) Accession No. FERM BP-6898 were inoculated into 50 mlof sterilized 23YT medium (16 g of tryptone, 10 g of yeast extract, 5 g of sodium chloride, and 1L of water, pH 7.0before sterillization) in a 500 ml Sakaguchi flask, and cultured with shaking at 37C for 18 hours. Then, 10 mg of1-chloro-3-hydroxyacetone, 70 mg of NAD+, and 25 mg of glucose were added to 1 ml of the resultant culture broth,and the mixture was then stirred at 30C for 2 hours. After the reaction was completed, the conversion rate and opticalpurity of the reaction product (S)-3-chloro-1,2-propanediol were analyzed by the same method as in Example 9. As aresult, the conversion rate was 42.8%, and the optical purity was 45.8%ee. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping