| 75% |
Stage #1: With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5 h;
Stage #2: With sulfuric acid; potassium iodide In water at 20℃; for 2 h; |
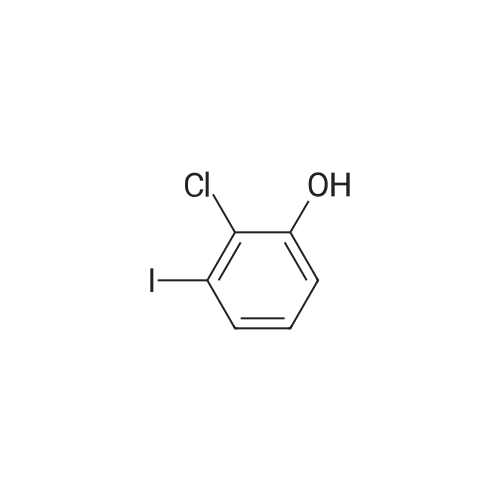
Example 10 [:] Synthesis of [2-CHLORO-3- (2, 2-DIMETHYL-4-PHENETHYLSULFANYLMETHYL-1,] 2- dihydroquinoline-6-yl) phenol OH z O < \\ CI 8r OrB/ H H Suzuki OH OH ci ci \\I \\I I/ \\ \\ I \\ N N H H 10 To a suspension of 2-amino-3-nitrophenol (10 g, 65 mmol) in concentrated [HC1] (10 mL) at [0°C,] sodium nitrite (5.1 g, 73.3 mmol) in water (60 [ML)] was added dropwise. After stirring for 30 minutes at [0°C,] CuCl (12.8 g, [130] mmol) in 10percent [H2SO4] (3 mL) was added and the reaction was stirred for 18 hours. The heterogeneous mixture was filtered and washed with water. The filtrate was extracted three times with 70 mL portions of EtOAc. Evaporation of EtOAc extractions afforded 7 grams (62percent yield) of pure [2-CHLORO-3-NITROPHENOL,] 2-Chloro-3- nitrophenol (6 g, 35 mmol) was dissolved in methanol (100 [ML)] and treated with ammonium chloride (9.4 g, 175.7 mmol) and zinc dust (46 g, 702.8 mmol). The resulting mixture was refluxed for one hour. After filtration and evaporation, 2-chloro-3-aminophenol was collected (4.5 g, 90percent yield) as a purple solid. To a suspension of 2-chloro-3-aminophenol (4.5 g, 31 mmol) in concentrated [HCI] (10 mL) at [0°C] was added dropwise sodium nitrite (2.5 g, 35 mmol) in water (50 mL). After stirring for 30 minutes at [0°C,] [I (I] (10.4 g, 63 mmol) in 10percent [H2SO4] (3 mL) was added dropwise. The reaction was stirred at room temperature for 2 hours, then filtered, extracted with EtOAc, and concentrated in vacuo to afford 2-chloro-3-iodophenol as a dark purple solid (6 g, 75percent yield). 6-Bromo-2,2, [4-TRIMETHYL-1,] 2-dihydroquinoline (2.3 g, 9.2 mmol) (Example 8) and bis (pinacol) diborane (4.2 g, 16.5 mmol) were combined in DMSO (2 mL). [KOAC] (2.7 g, 27.4 mmol) was added, followed by [PDCL2] (dppf) (100 mg). The contents were placed into a microwave reaction vessel assembly and irradiated at [120°C] for 15 minutes. After cooling to room temperature, purification by chromatography afforded 2,2, [4-TRIMETHYL-6- (4,] 4,5, 5- tetramethyl- [1, 3,2] [DIOXABOROLAN-2-YL)-1,] 2-dihydroquinoline (2.3 g, [84percent] yield) as a white crystalline solid. 2,2, [4-TRIMETHYL-6- (4,] 4,5, [5-TETRAMETHYL- [1,] 3,2] [DIOXABOROLAN-2-YL)-1,] 2-dihydroquinoline [(308] mg) and 393 mg of 2-chloro-3-iodophenol were coupled using of 100 mg [OF PDCL2 (DPPF)] and [300 MG OF KOAC TO GIVE 250 MG OF 2-CHLORO-3- (2, 2, 4-TRIMETHYL-1, 2-DIHYDROQUINOLIN-6-YL) -] phenol. Using the procedures described in Example 7, this (85 mg) was reacted with NBS to give 56 mg [OF 3- (4-BROMOMETHYL-2, 2-DIMETHYL-1, 2-DIHYDROQUINOLIN-6-YL)-2-CHLOROPHENOL.] The product (50 mg) was coupled with 0.04 mL of 2-phenylethanethiol to give 40 mg of the title compound as an oil. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping