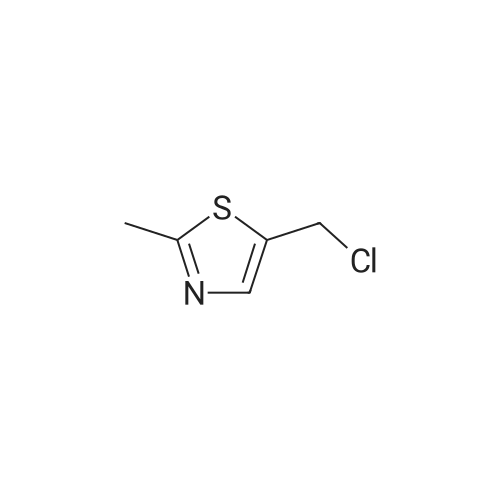
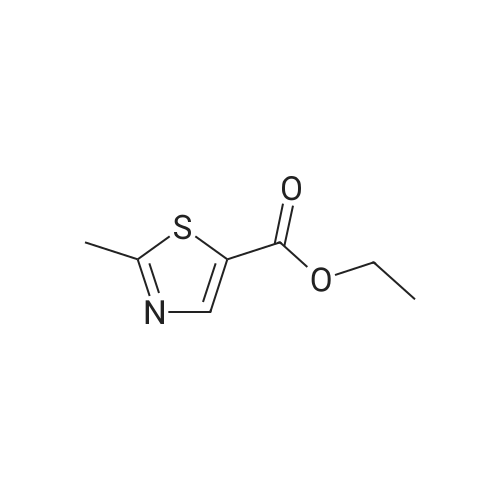
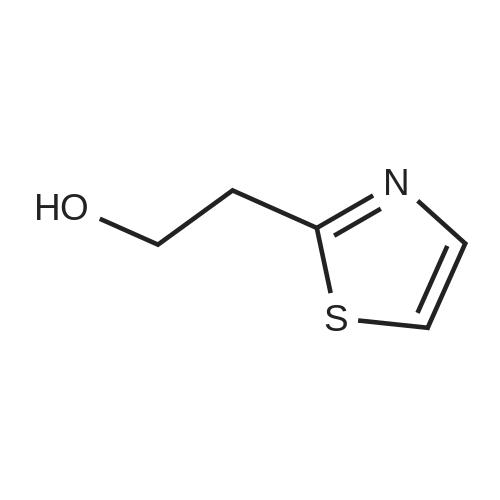
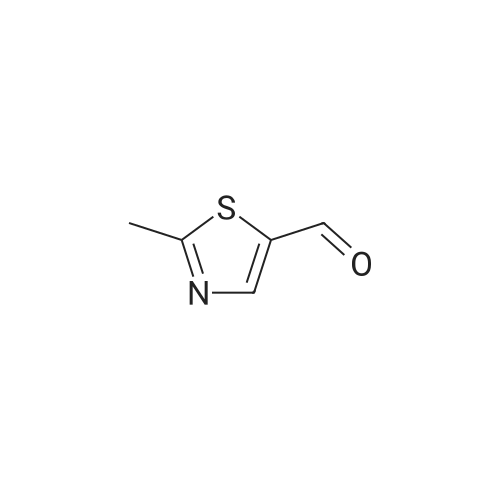
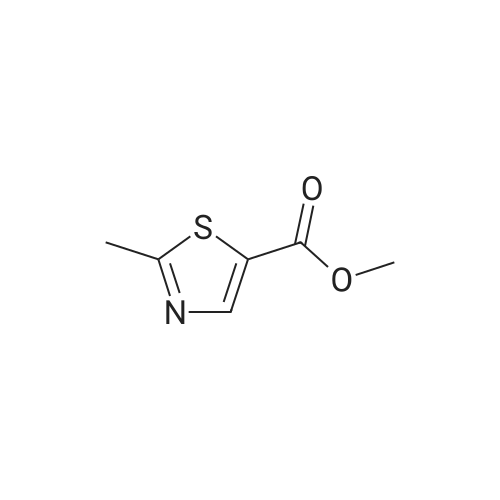
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; for 2h; |
To a solution of intermediate 7-c (22.2 g, 130.0 mmol) in THF (430 ml) cooled to 0°C was added a 1.0 M solution LiAIH4 in THF (91.0 ml, 91.0 mmol). The solution was slowly warmed to room temperature and stirred for 2 hours. Water (3.5 ml) was slowly added, followed by 3.5 ml 15percent NaOH (3.5 ml) and water (10.5 ml) and the mixture was stirred for 1 hour. The reaction was filtered through celite and the filtrate collected. Volatiles were removed in vacuo to provide intermediate 7-d as a yellow oil. |
|
|
Step 3: Intermediate 5-d; To a solution of intermediate 5-c (22.2 g, 130.0 mmol) in THF (430 ml) cooled to 0°C was added a 1.0 M solution LiAIH4 in THF (91.0 ml, 91.0 mmol) and the solution was slowly warmed to room temperature and stirred for 2 hours. Water (3.5 ml) was slowly added, followed by 3.5 ml lSpercent NaOH (3.5 ml) and water (10.5 ml) and the mixture was stirred for 1 hour. The reaction was filtered over celite and volatiles were removed under reduced pressure to provide intermediate 5-d as yellow oil. |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃;Inert atmosphere; |
To a stirred solution of ethyl 2-methylthiazole-5-carboxylate (1 eq) in dry THF (5 mL) at 0 <C ) under N2 was added LiAIH4 (1 .1 eq., 2.0 M solution in THF) dropwise. The reaction mixture was stirred at RT for 1 h. The reaction progress was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to -10°C to 0 < and then quenched by the dropwise addition of 10percent NaOH aqueous solution (5 mL). After 10 min of stirring, the mixture was filtered through a pad of Celite and the filtrate was concentrated under reduced pressure to afford (2-methylthiazol-5-yl)methanol (6 g) as a pale yellow solid. The crude product used in the next step without purification. LC/MS: (Method A) 130.0 (M+H). H NMR (DMSO-de, 400 MHz): delta 7.4 (s, 1 H), 5.5 (s, 1 H), 4.6 (d, J = 4.0 Hz, 2H), 2.6 (s, 3H). |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; for 2h; |
Step 4: Intermediate 29-d [0172] To a solution of intermediate 29-c (22.2 g, 130.0 mmol) in THF (430 ml) cooled to 0° C. was added a 1.0 M solution of LiAlH4 in THF (91.0 ml, 91.0 mmol) and the solution was slowly warmed to room temperature and stirred for 2 hours. Water (3.5 ml) was slowly added, followed by 3.5 ml 15percent NaOH (3.5 ml) and water (10.5 ml) and the mixture was stirred for 1 hour. The reaction was filtered over celite and volatiles were removed in vacuo to provide intermediate 29-d as yellow oil. |
| 12g |
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 20℃; for 18h; |
Step 1: Synthesis of 2-methyl-5-hydroxymethylthiazole: At room temperature, lithium aluminum hydride (8.88g, 234mmol) was dispersed in anhydrous tetrahydrofuran (THF, 100mL). The resulting mixture was cooled to 0-5°C in an ice-water bath. To the cooled mixture was dropwise added a solution of 2-methyl-5-ethoxyformylthiazole (20g,117mmol) in anhydrous tetrahydrofuran (THF, 100mL). After the completion of the dropwise addition, the reaction mixture was naturally warmed to room temperature, and was stirred for 18 hours at room temperature. To the reaction mixture was dropwise added water (10mL) at 0-5°C. After the completion of the dropwise addition, the resulting mixture was filtered. The filtrate was concentrated to produce a yellow oily substance (12g), which was directly used in the next step. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping