| 87% |
With sodium hydroxide; In tetrahydrofuran; water; at 0 - 20℃; for 16h; |
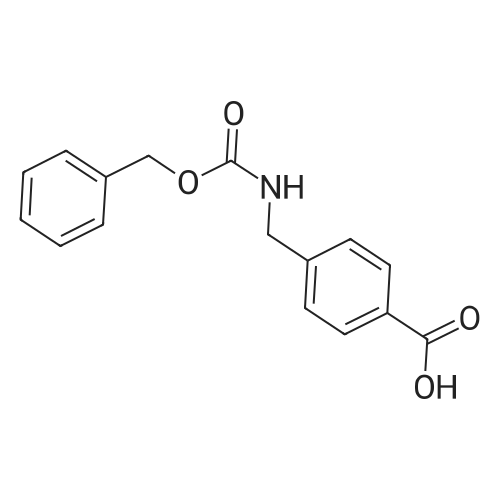
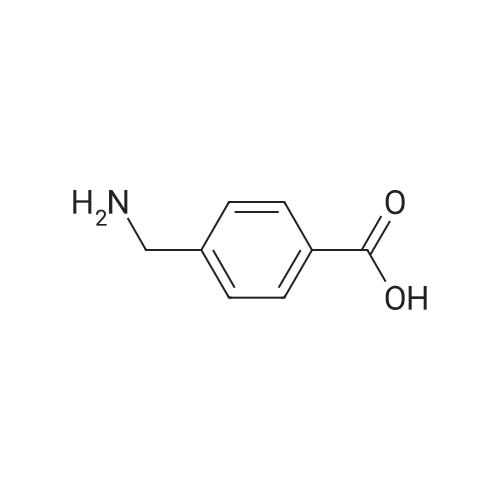
Benzyl chloroformate (10.3 mL, 72.7 mmol) and 2 M NaOH solution (33 mL, 66 mmol) were simultaneously added dropwise to a stirred solution of 4-aminomethylbenzoic acid (29) (10.0 g, 66.2 mmol) in 2 M NaOH solution (33 mL) and THF (30 mL) at 0 C. The mixture was stirred at 20 C for 16 h, then the organic solvent was evaporated and the residue acidified with 2 M HC1 until the pH of the mixture was 2-3. The precipitate was filtered, washed with water (250 mL), washed with EtOH (50 mL), and finally washed with Et20 (100 mL). The solid was dried under vacuum to give acid 2 (16.43 g, 87%) as a white powder: mp 190-192 C [lit. (Loge et. al., J. Enzyme Inhibit. Med. Chem. 2002, 17, 381-390) mp (toluene) 194-195 C; 1H NMR δ 7.85 (br d, 2 H, H-2, H-6), 7.82 (br t, J = 6.1 Hz, 1 H, NHC02), 7.30-7.40 (m, 5 H, H-2', H-3', H-4', H-5', H-6'), 7.27 (br d, J = 8.2 Hz, 2 H, H-3, H-5), 5.05 (s, 2 H, OCH2), 4.24 (d, J = 6.1 Hz, 2 H, CH2N). |
| 87% |
With sodium hydroxide; In tetrahydrofuran; at 0 - 20℃; for 16h; |
Benzyl chloroformate (10.3 mL, 72.7 mmol) and 2 M NaOH solution (33 mL, 66 mmol) were simultaneously added dropwise to a stirred solution of 4-aminomethylbenzoic acid (29) (10.0 g, 66.2 mmol) in 2 M NaOH solution (33 mL) and THF (30 mL) at 0 C. The mixture was stirred at 20 C. for 16 h, then the organic solvent was evaporated and the residue acidified with 2 M HCl until the pH of the mixture was 2-3. The precipitate was filtered, washed with water (250 mL), washed with EtOH (50 mL), and finally washed with Et2O (100 mL). The solid was dried under vacuum to give acid 2 (16.43 g, 87%) as a white powder: mp 190-192 C. [lit. (Loge et. al., J. Enzyme Inhibit. Med. Chem. 2002, 17, 381-390) mp (toluene) 194-195 C.; 1H NMR δ 7.85 (br d, 2H, H-2, H-6), 7.82 (br t, J=6.1 Hz, 1H, NHCO2), 7.30-7.40 (m, 5H, H-2', H-3', H-4', H-5', H-6'), 7.27 (br d, J=8.2 Hz, 2H, H-3, H-5), 5.05 (s, 2H, OCH2), 4.24 (d, J=6.1 Hz, 2H, CH2N). |
| 87% |
With sodium hydroxide; In tetrahydrofuran; at 0 - 20℃; for 16h; |
Benzylchloroformate (10.3 mL, 72.7 mmol) and 2 M NaOH solution (33 mL, 66 mmol) weresimultaneously added dropwise to a stirred solution of 4-aminomethylbenzoicacid (1) (10.0 g, 66.2 mmol) in 2 M NaOH solution (33 mL) and THF (30 mL) at 0C. The mixture was stirred at 20 C. for 16 h, then the organic solvent wasevaporated and the residue acidified with 2 M HCl until the pH of the mixturewas 2-3. The precipitate was filtered, washed with water (250 mL), washed withEtOH (50 mL), and finally washed with Et2O (100 mL). The solid was dried undervacuum to give acid 2 (16.43 g, 87%) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping