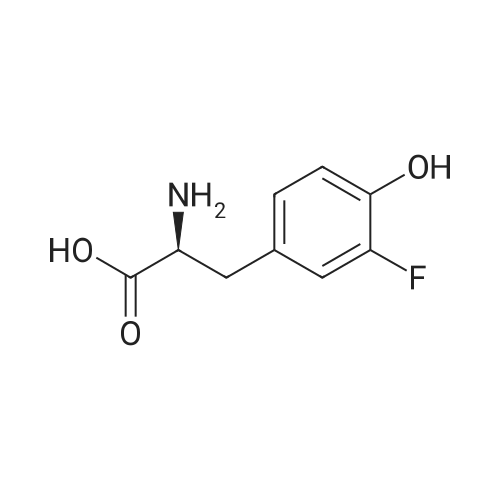
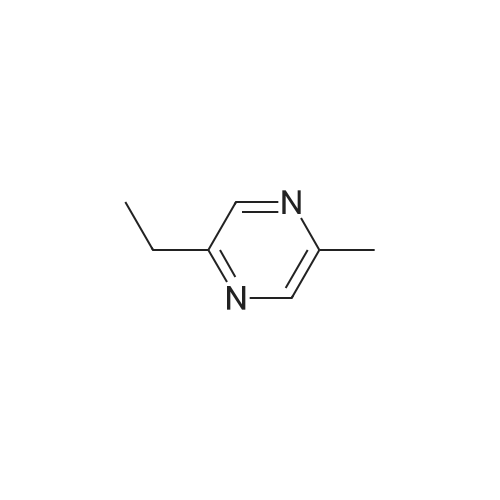
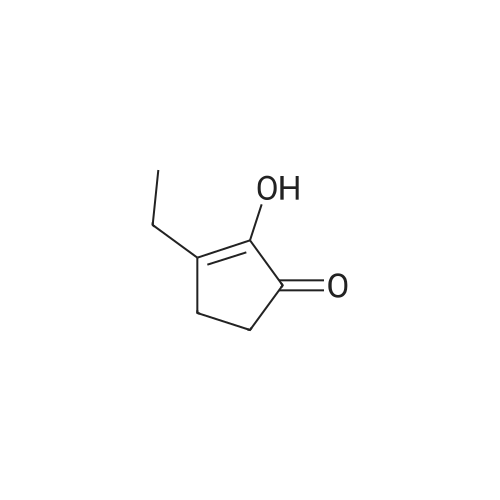
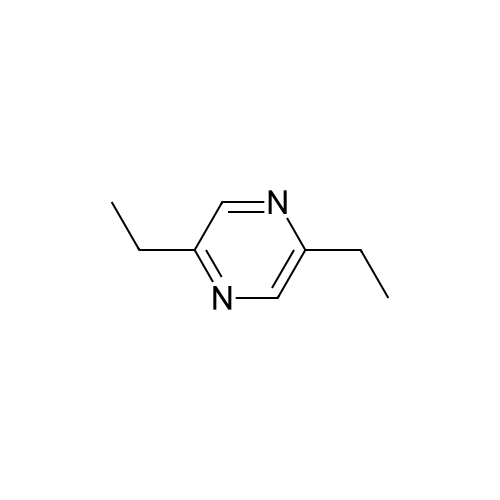
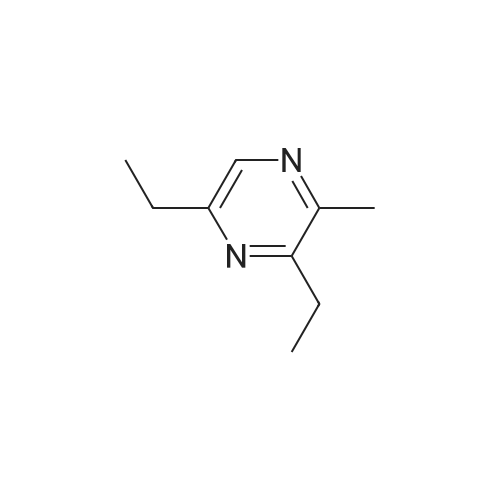
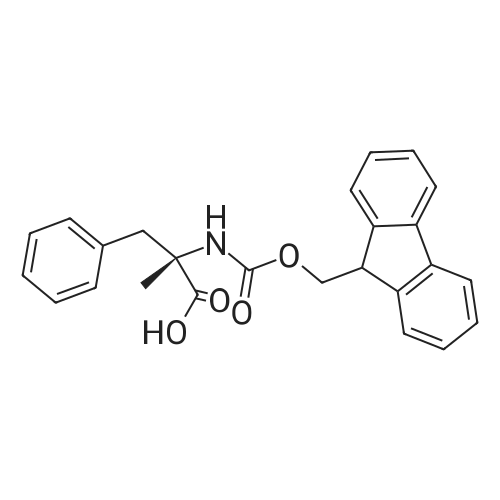
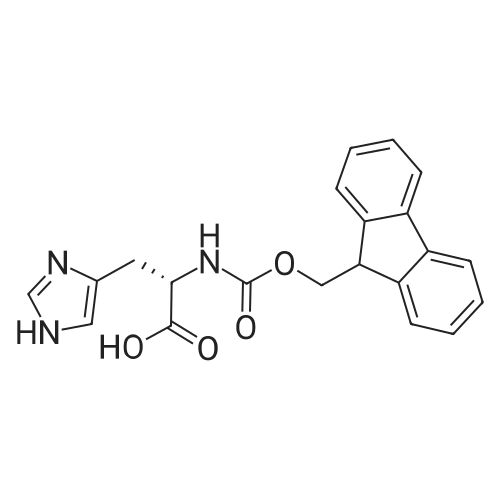
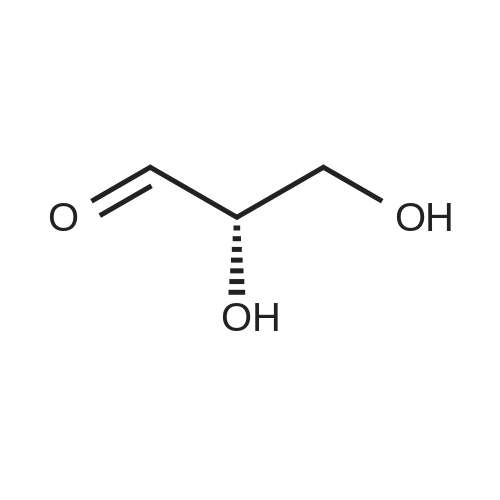
| 65% |
Stage #1: With bromic acid; potassium bromide; sodium nitrite In water at -10 - 20℃;
Stage #2: With bromic acid In water at 65℃; for 48 h; |
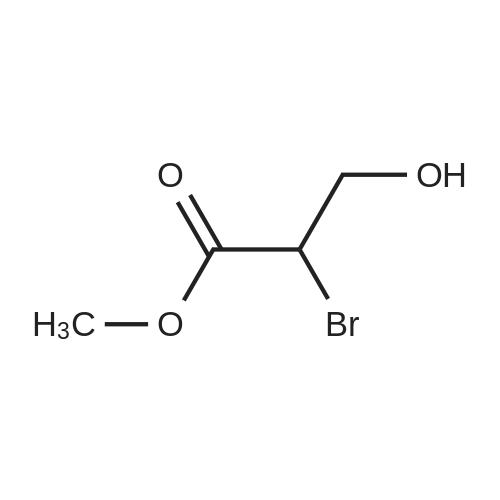
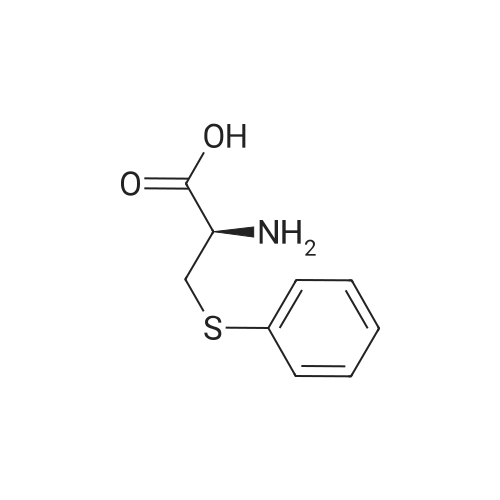
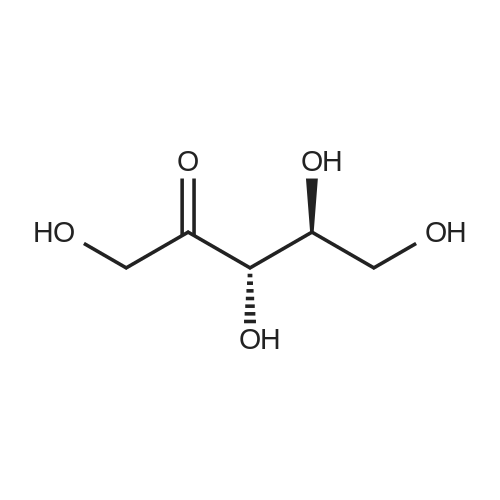
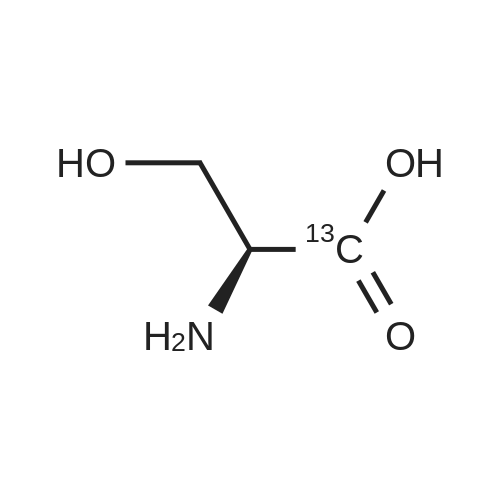
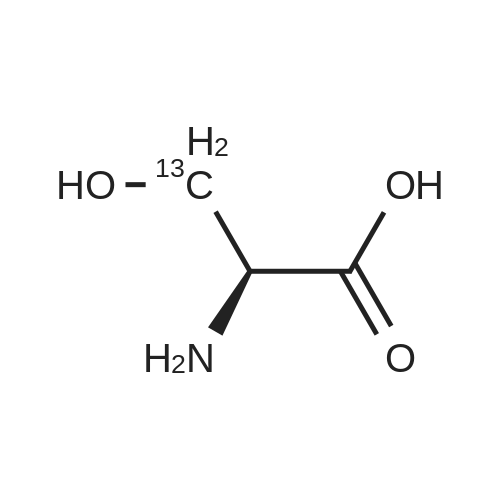
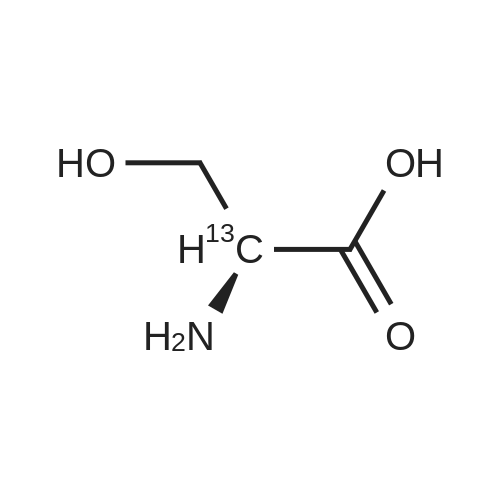
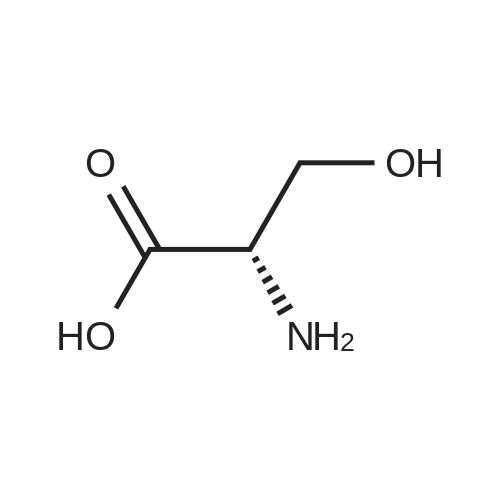
[1496] Step 2: methyl 2-bromo-3-hydroxypropanoate[1497] To a solution of L-serine (5.0 g, 47.6 mmol), a 48 w/wpercent bromic acid solution (13.0 mL, 109.5 mmol), and potassium bromide (20.0 g, 157.1 mmol) in water (44.0 mL) was added portionwise sodium nitrite (6.0 g, 80.9 mmol) at -10 ℃. The reaction mixture was stirred at room temperature overnight. The reaction mixture was saturated with sodium chloride and then extracted with ethyl acetate. The organic layer was dried over anhydrous magnesium sulfate, filtered, and then evaporated. The residue was dissolved in methanol (50.0 mL) and then 48 w/wpercent bromic acid (0.2 mL) was added thereto. The reaction mixture was stirred at 65 ℃ for 2 days and then concentrated under reduced pressure to discard excess methanol. The resulting dark yellow residue was dissolved in dichloromethane (100.0 mL) and then washed with a saturated sodium hydrogen carbonate solution (50.0 mL) and brine. The organic layer was dried over anhydrous sodium sulfate, filtered, and then evaporated. The residue was dried under reduced pressure to give 5.7 g of the titled compound (Yield: 65percent).[1498] 1H NMR (CDCl3, 400 MHz) δ 4.36(t, 1H), 4.01-4.08(m, 1H), 3.93-3.97(m, 1H), 3.82(s, 3H), 2.56(t, 1H) |
| 65% |
Stage #1: With bromic acid; potassium bromide; sodium nitrite In water at -10 - 20℃;
Stage #2: at 65℃; for 48 h; |
Step 2:
methyl 2-bromo-3-hydroxypropanoate
To a solution of L-serine (5.0 g, 47.6 mmol), a 48 w/w percent bromic acid solution (13.0 mL, 109.5 mmol), and potassium bromide (20.0 g, 157.1 mmol) in water (44.0 mL) was added portionwise sodium nitrite (6.0 g, 80.9 mmol) at -10° C.
The reaction mixture was stirred at room temperature overnight.
The reaction mixture was saturated with sodium chloride and then extracted with ethyl acetate.
The organic layer was dried over anhydrous magnesium sulfate, filtered, and then evaporated.
The residue was dissolved in methanol (50.0 mL) and then 48 w/w percent bromic acid (0.2 mL) was added thereto.
The reaction mixture was stirred at 65° C. for 2 days and then concentrated under reduced pressure to discard excess methanol.
The resulting dark yellow residue was dissolved in dichloromethane (100.0 mL) and then washed with a saturated sodium hydrogen carbonate solution (50.0 mL) and brine.
The organic layer was dried over anhydrous sodium sulfate, filtered, and then evaporated.
The residue was dried under reduced pressure to give 5.7 g of the titled compound (Yield: 65percent).
1H NMR (CDCl3, 400 MHz) δ 4.36 (t, 1H), 4.01-4.08 (m, 1H), 3.93-3.97 (m, 1H), 3.82 (s, 3H), 2.56 (t, 1H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping