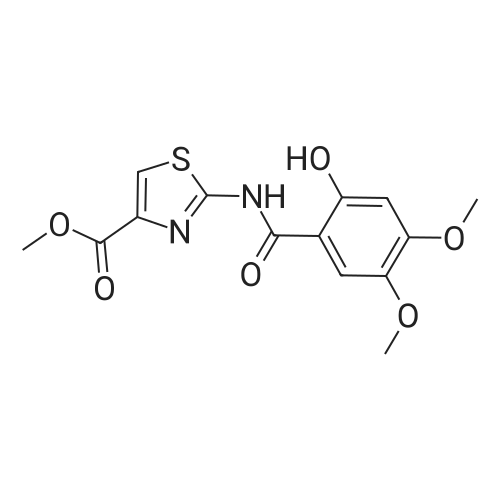
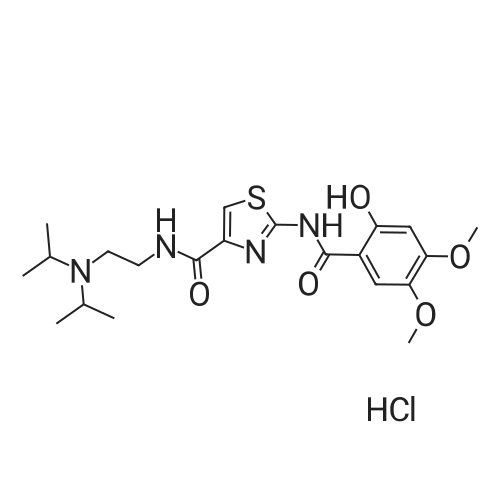
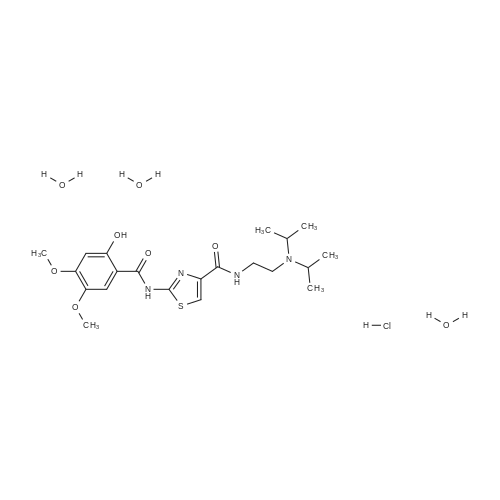
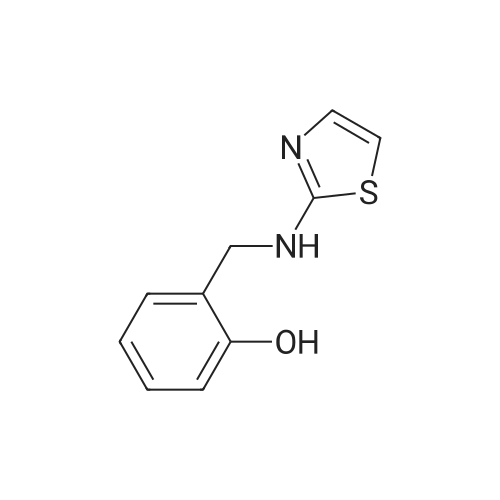
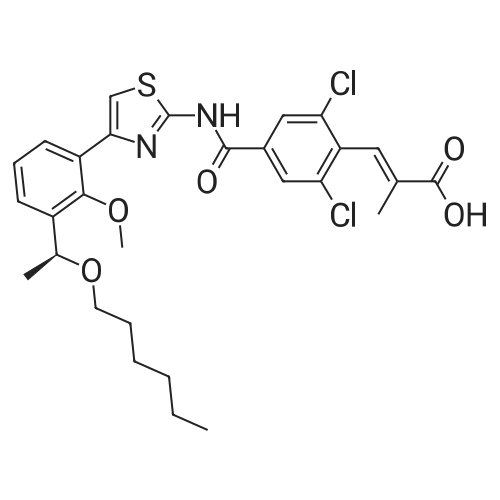
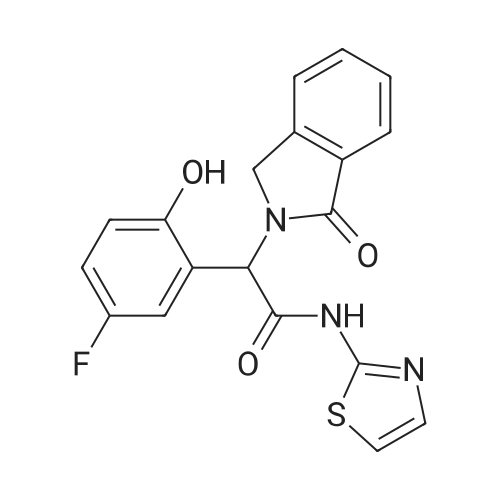
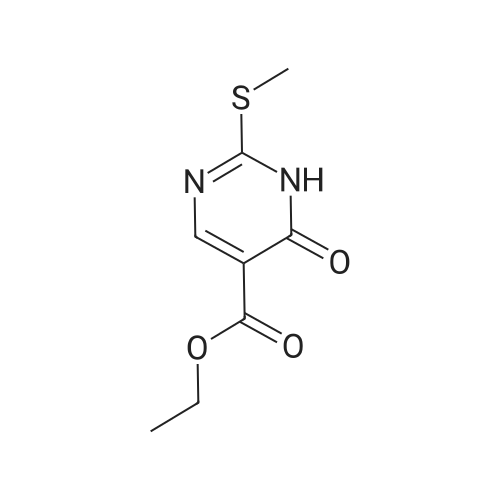
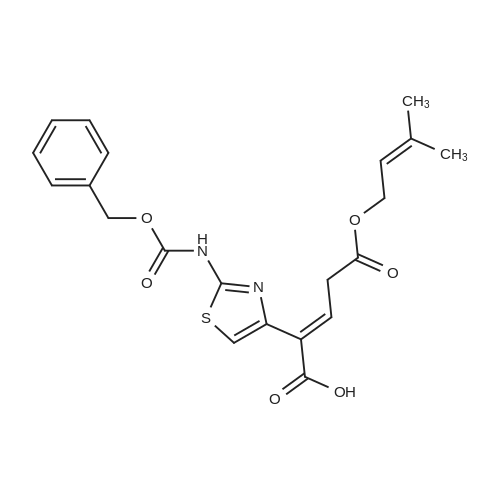
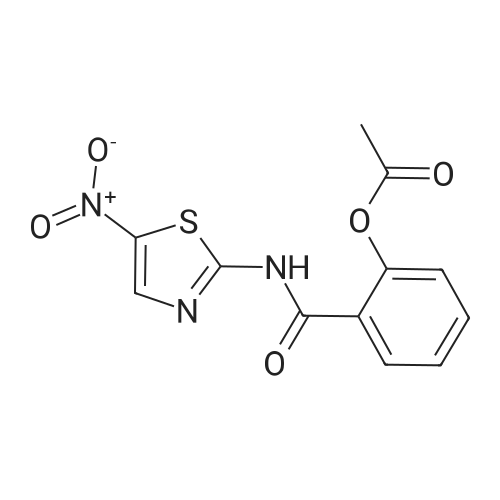
Synthesis and In Vitro Antibacterial Evaluation of Mannich Base Nitrothiazole Derivatives
Phelelisiwe S. Dube
;
Dylan Hart
;
Lesetja J. Legoabe
, et al.
Molbank,2024,2024(1):M1793.
DOI:
10.3390/M1793
More
Abstract: Nitrothiazole derivatives have been reported to exhibit activity against aerobic, anaerobic, and microaerophilic bacteria. This activity profile makes the nitrothiazole compound class an ideal lead source against Mycobacterium tuberculosis, which flourishes in varied environments with different oxygen concentrations. In this work, we investigated six nitrothiazole derivatives for antitubercular activity. The compounds exhibited potent activity, with compounds 9 and 10 possessing an equipotent MIC90 value of 0.24 μM. The compounds were investigated for cytotoxicity against HEK293 cells and hemolysis against red blood cells, and they demonstrated no cytotoxicity nor hemolytic effects, suggesting they possess inherent antitubercular activity.
Keywords:
nitrothiazole ;
Mannich bases ;
antitubercular activity ;
tuberculosis ;
Mycobacterium tuberculosis
Purchased from AmBeed:
121-66-4 ;
55981-09-4 ;
51322-75-9

Need for a Standardized Translational Drug Development Platform: Lessons Learned from the Repurposing of Drugs for COVID-19
Frauke Assmus
;
Jean-Sélim Driouich
;
Rana Abdelnabi
, et al.
Microorganisms,2022,10(8):1639.
DOI:
10.3390/microorganisms10081639
PubMed ID:
36014057
More
Abstract: In the absence of drugs to treat or prevent COVID-19, drug repurposing can be a valuable strategy. Despite a substantial number of clinical trials, drug repurposing did not deliver on its promise. While success was observed with some repurposed drugs (e.g., remdesivir, dexamethasone, tocilizumab, baricitinib), others failed to show clinical efficacy. One reason is the lack of clear translational processes based on adequate preclinical profiling before clinical evaluation. Combined with limitations of existing in vitro and in vivo models, there is a need for a systematic approach to urgent antiviral drug development in the context of a global pandemic. We implemented a methodology to test repurposed and experimental drugs to generate robust preclinical evidence for further clinical development. This translational drug development platform comprises in vitro, ex vivo, and in vivo models of SARS-CoV-2, along with pharmacokinetic modeling and simulation approaches to evaluate exposure levels in plasma and target organs. Here, we provide examples of identified repurposed antiviral drugs tested within our multidisciplinary collaboration to highlight lessons learned in urgent antiviral drug development during the COVID-19 pandemic. Our data confirm the importance of assessing in vitro and in vivo potency in multiple assays to boost the translatability of pre-clinical data. The value of pharmacokinetic modeling and simulations for compound prioritization is also discussed. We advocate the need for a standardized translational drug development platform for mild-to-moderate COVID-19 to generate preclinical evidence in support of clinical trials. We propose clear prerequisites for progression of drug candidates for repurposing into clinical trials. Further research is needed to gain a deeper understanding of the scope and limitations of the presented translational drug development platform.
Keywords:
COVID-19 ;
drug repurposing ;
translational medicine ;
pandemics ;
clinical trials
Purchased from AmBeed:
55981-09-4 ;
23828-92-4 ;
198904-31-3 ;
59721-29-8 ;
259793-96-9 ;
1190307-88-0 ;
481-49-2 ;
155213-67-5 ;
61718-82-9


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping