|
With potassium phosphate; copper(l) iodide; trans-1,2-cyclohexanediamine; In 1,4-dioxane; at 100℃; for 12.0h; |
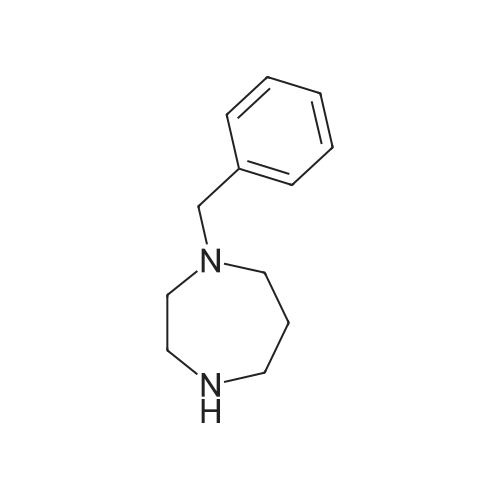
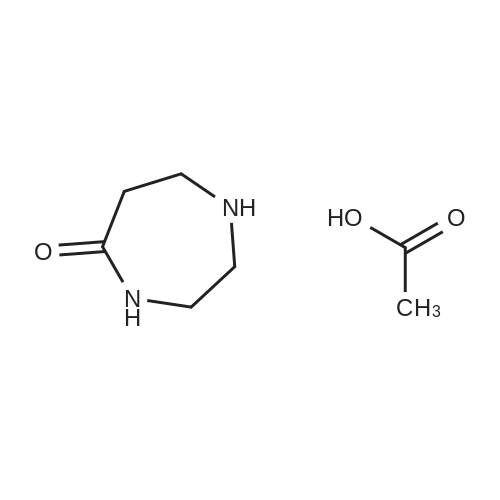
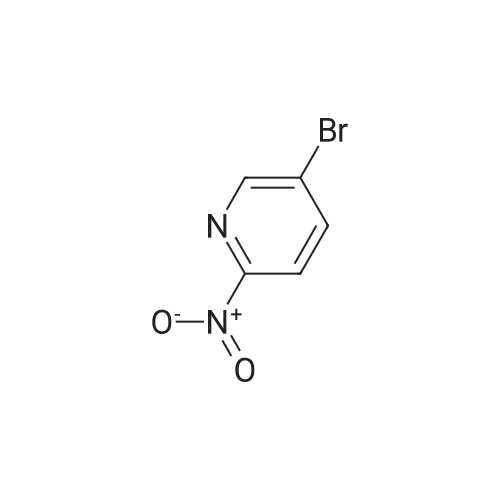
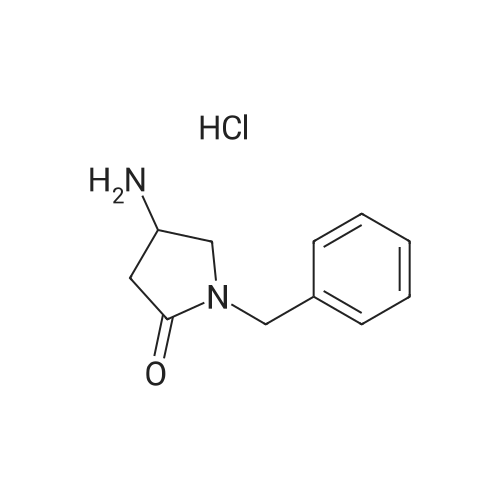
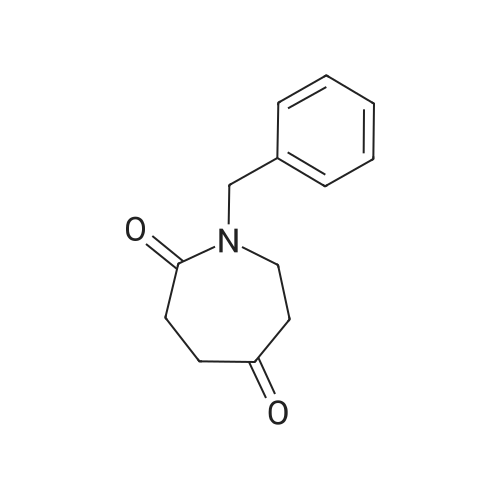
To a solution of 24.6 mmol of 9 and 27.3 mmol of 7 in 50 mL of 1,4-dioxane was added 4.92 mmol of copper (I) iodide followed by the addition of 49.2 mmol of K3P04 and 4.92 mmol of trans-cyclohexanediamine, then the resulting mixture was stirred at 100C for 12 h. The reaction mixture was cooled to room temperature and concentrated in vacuo. The residue was diluted with CHC13, poured into water, and insoluble material was removed by celite filtration. The filtrate was extracted with CHC13, dried over MgS04 and concentrated in vacuo. The crude product was purified by column chromatography to give 7.87 mmol of nitro derivative. [0202] A solution of 7.66 mmol of nitro derivative and 0.5 g of Pd/C (10%) in 150 mL of methanol was stirred overnight under H2 (1 atm). After filtering through celite, the solution was concentrated under reduced pressure to give 4.75 mmol of 8. |
|
With potassium phosphate;copper(l) iodide; trans-1,2-cyclohexanediamine; In 1,4-dioxane; at 100℃; for 12.0h; |
To a solution of 24.6 mmol of 9 and 27.3 mmol of 7 in 50 mL of 1,4-dioxane was added 4.92 mmol of copper (I) iodide followed by the addition of 49.2 mmol ofK3P04 and 4.92 mmol of trans-cyclohexanediamine, then the resulting mixture was stirred at 100C for 12 h. The reaction mixture was cooled to room temperature and concentrated in vacuo. The residue was diluted with CHC13, poured into water, and insoluble material was removed by celite filtration. The filtrate was extracted with CHCl3, dried over MgS04 and concentrated in vacuo. The crude product was purified by column chromatography to give 7.87 mmol of nitro derivative. [0233] A solution of 7.66 mmol of nitro derivative and 0.5 g of Pd/C (10%) in 150 mL of methanol was stirred overnight under H2 (1 atm). After filtering through celite, the solution was concentrated under reduced pressure to give 4.75 mmol of 8. 1H NMR (400 MHz, DMSO-d6) 8 7.70 (d, J = 2.4 Hz, 1H), 7.17 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 7.30-7.36 (m, 5H), 6.40 (d, J = 8.8 Hz, 1H), 5.90 (br s, 2H), 3.66-3.72 (m, 2H), 3.59 (br s, 2H), 2.59-2.71 (m, 6H); MS m/z: 327 (M+I). |
|
With potassium phosphate; copper(l) iodide; trans-1,2-cyclohexanediamine; In 1,4-dioxane; at 100℃; for 12.0h; |
Example 6 Preparation of 8 6.1 Ullmann Cross-Coupling To a solution of 24.6 mmol of 9 and 27.3 mmol of 7 in 50 mL of 1,4-dioxane was added 4.92 mmol of copper (I) iodide followed by the addition of 49.2 mmol of K3PO4 and 4.92 mmol of trans-cyclohexanediamine, then the resulting mixture was stirred at 100 C. for 12 h. The reaction mixture was cooled to room temperature and concentrated in vacuo. The residue was diluted with CHCl3, poured into water, and insoluble material was removed by celite filtration. The filtrate was extracted with CHCl3, dried over MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography to give 7.87 mmol of nitro derivative. A solution of 7.66 mmol of nitro derivative and 0.5 g of Pd/C (10%) in 150 mL of methanol was stirred overnight under H2 (1 atm). After filtering through celite, the solution was concentrated under reduced pressure to give 4.75 mmol of 8. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping