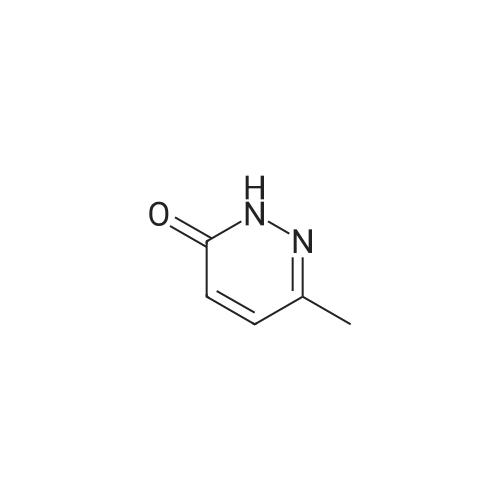
| 77% |
With dipyridinium dichromate; sulfuric acid; at 50 - 60℃; for 0.5h; |
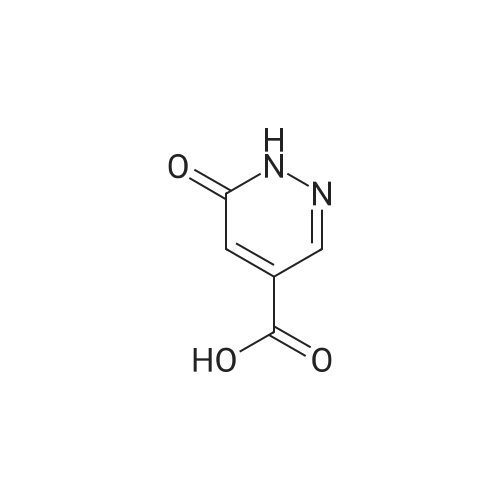
Step 14B: 6-Oxo-1H-pyridazine-4-carboxylic acid; To a stirred solution of the subtitle compound of Step 14A (4.4 g, 40 mmol) in concentrated sulphuric acid (80 mL), potassium dichromate (18 g, 61 mmol) was added in small quantities at 50-60 C. as a finely ground powder. The starting material was added to the mixture within 20 min. Stirring was continued for a further 10 min at 60 C., then the viscous green mixture was poured on crushed ice. The solid powder, which separated, was collected, washed with cold water and dried to give the subtitle compound (4.5 g, 77%).1H NMR (400 MHz, (CD3)2SO): delta 7.22 (s, 3H), 8.13 (s, 1H), 13.38 (s, broad, 1H). |
| 77% |
With potassium dichromate; sulfuric acid; at 50 - 60℃; for 0.5h; |
Step 10B: 6-Oxo-1,6-dihydropyridazine-4-carboxylic acid; To a stirred solution of the subtitle compound of Step 10A (4.4 g, 40 mmol) in concentrated sulphuric acid (80 mL), potassium dichromate (18 g, 61 mmol) was added in small quantities at 50-60 C. as a finely ground powder. The starting material was added to the mixture within 20 min. Stirring was continued for 10 min at 60 C., the viscous green mixture was poured on crushed ice. The solids were filtered off and washed with cold water. After drying in vacuo the subtitle compound was isolated (4.5 g, 77%).1H NMR (400 MHz, DMSO-d6): delta 7.22 (s, 3H), 8.13 (s, 1H), 13.38 (s, broad, 1H). |
| 77% |
With potassium dichromate; sulfuric acid; at 50 - 60℃; for 0.5h; |
Step 7B: 6-Oxo-1,6-dihydropyridazine-4-carboxylic Acid To a stirred solution of the subtitle compound of Step 7A (4.4 g, 40 mmol) in concentrated sulphuric acid (80 mL), potassium dichromate (18 g, 61 mmol) was added in small quantities at 50-60 C. as a finely ground powder. The starting material was added to the mixture within 20 min. Stirring was continued for a further 10 min at 60 C., then the viscous green mixture was poured on crushed ice. The solid powder, which separated, was collected, washed with cold water and dried to give the subtitle compound (4.5 g, 77%).1H NMR (400 MHz, (CD3)2SO): delta 7.22 (s, 3H), 8.13 (s, 1H), 13.38 (s, broad, 1H). |
| 77% |
With potassium dichromate; sulfuric acid; at 60℃; for 0.166667h; |
Step 13B: 6-Oxo-1,6-dihydropyridazine-4-carboxylic acid To a stirred solution of the subtitle compound of Step 13A (4.4 g, 40 mmol) in concentrated sulphuric acid (80 mL), potassium dichromate (18 g, 61 mmol) was added in small quantities at 50-60 C. as a finely ground powder. The starting material was added to the mixture within 20 min. Stirring was continued for a further 10 min at 60 C., the viscous green mixture was poured on crushed ice. The solids were filtered off and washed with cold water. After drying in vacuo the subtitle compound was isolated (4.5 g, 77%). 1H NMR (400 MHz, (CD3)2SO): delta 7.22 (s, 3H), 8.13 (s,1H), 13.38 (s, broad, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping