| 89% |
Stage #1: at 100℃; for 6 h;
Stage #2: at 20℃; for 20 h; |
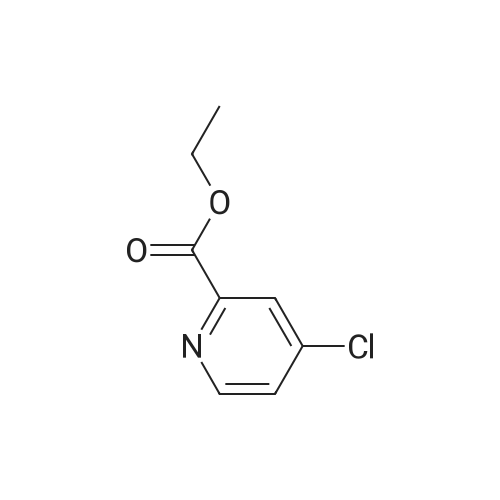
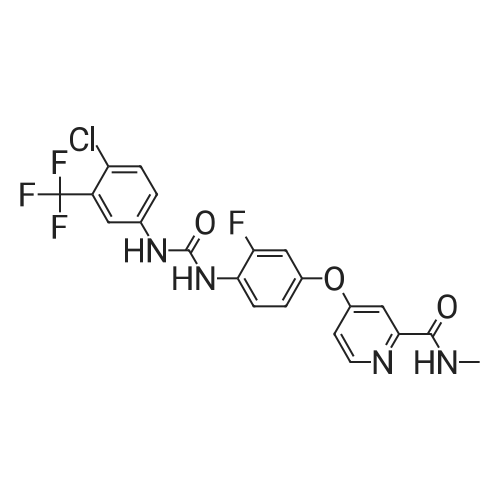
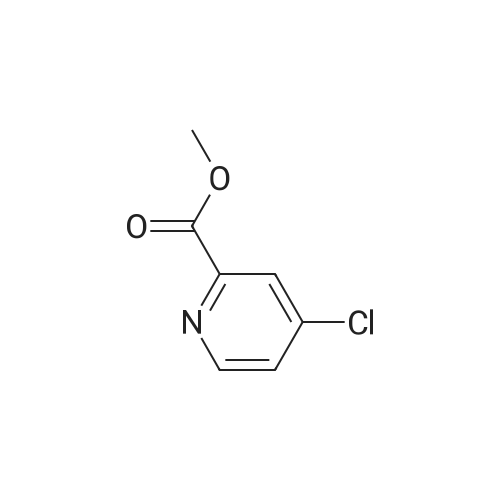
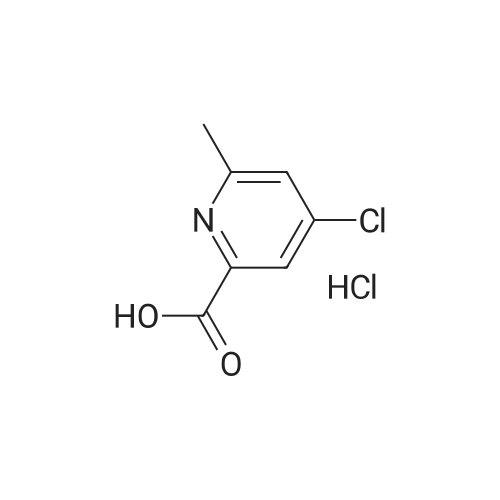
Step-1 : Ethyl-4-chloroypicolinate: A mixture of 4-chloropicolinic acid (20 g, 127 mmol) and thionyl chloride (200 mL) was heated to 100 °C and maintained for 6 h. The reaction was cooled to RT, and the excess of thionyl chloride was removed under vacuum. To the above obtained residue was then added ethanol (200 mL) at 0 °C drop-wise and the resulting mixture was stirred at RT for 20 h.The solvent was evaporated under vacuum and the residue was taken in ethyl acetate (1000 mL), washed with water (2x500 mL), saturated sodium bicarbonate solution (2x500 mL), brine (500 mL), dried (Na2S04) and filtered. The filtrate was evaporated to yield 21.0 g (89percent) the desired product as a semi solid. XHNMR (400 MHz, DMSO) δ 8.66 (d, = 5.0 Hz, 1Η), 8.13 (d, = 2.0Hz, 1Η), 7.49 (dd, = 5.0 &2.0HZ 1Η), 4.47 (q, = 7.0 Hz, 2Η), 1.46 (t, = 7.0 Hz, 3H); GC-MS (m/z) 185, 187 [(M)+, CI35' 37]. |
| 85% |
Stage #1: at 100℃; for 6 h;
Stage #2: at 0 - 20℃; for 8 h; |
Step 1:
Preparation of ethyl 4-chloropicolinate
To 4-chloropicolinic acid (1.0 g, 6.35 mmol) was added SOCl2 (10 mL) at RT.
The reaction mixture was heated at 100° C. for 6 h then concentrated to remove excess SOCl2.
To the resulting residue was added EtOH (10 mL) dropwise at 0° C. and the reaction mixture was stirred at RT for 8 h.
After completion, the reaction mixture was concentrated and diluted with EtOAc (50 mL).
The organic phase was washed with water (2*25 mL), saturated sodium bicarbonate solution (2*25 mL) and brine (25 mL).
The organic layer was dried over sodium sulfate and concentrated to afford ethyl 4-chloropicolinate (1.0 g, 85percent).
1H NMR (400 MHz, DMSO-d6) δ 8.71-8.72 (d, J=8.8 Hz, 1H), 8.09 (s, 1H), 7.84-7.85 (m, 1H), 4.34-4.39 (q, J=7.2 Hz, 2H), 1.33-1.36 (t, 3H). |
| 83.6% |
Stage #1: at 100℃; for 6 h; Inert atmosphere
Stage #2: at 20℃; for 25.5 h; Cooling with ice
Stage #3: With sodium hydrogencarbonate In water |
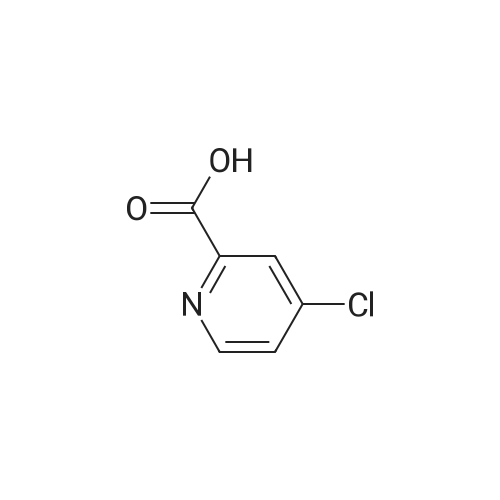
(Reference Example A-1) Ethyl 4-chloropyridine-2-carboxylate
A mixture of 4-chloropyridine-2-carboxylic acid (39.4g) and thionyl chloride (64 ml) was heated and stirred at 100 °C under a nitrogen atmosphere for 6 hr.
The reaction mixture was allowed to cool down to room temperature.
This was concentrated under reduced pressure and distilled azeotropically with toluene.
The resultant residue was gradually added to ethanol while stirring in an ice bath.
The reaction mixture was stirred at room temperature for 25.5 hr.
The reaction mixture was concentrated under reduced pressure.
To the residue was added a saturated aqueous solution of sodium hydrogencarbonate and extracted with ethyl acetate.
The organic layer was washed with brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to provide the titled compound as a brown oil (38.8 g, 83.6 percent).
1H-NMR Spectrum (CDCl3) δ (ppm): 1.46 (3H, t, J = 7.2 Hz), 4.50 (2H, q, J = 7.2 Hz), 7.49 (1H, dd, J = 2.0, 5.2 Hz), 8.15 (1H, d, J = 2.0 Hz), 8.67 (1H, d, J = 5.2 Hz).
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping