|
With pyridine; In dichloromethane; at 5 - 20℃; for 2.16667h; |
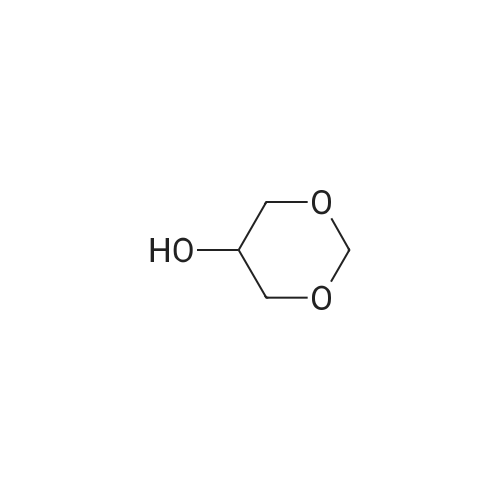
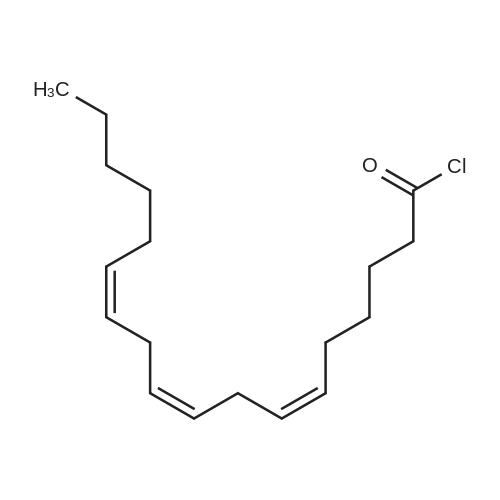
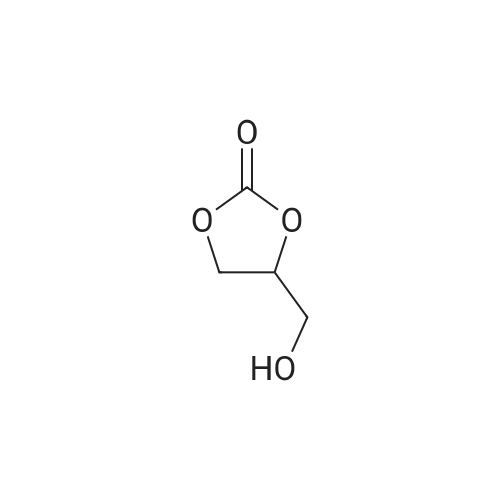
EXAMPLE 2.; 2-GLA <strong>[4740-78-7]glycerol formal</strong> (aka: 5-y-linolenoyloxy)-l,3-dioxan and 5-d,3-dioxanvD- methyl-gamma-linolenate); For the purposes of this exemplification, the synthesis of this ester was carried out by acylation of the commercially available <strong>[4740-78-7]glycerol formal</strong> mixture using gamma-linolenoyl chloride. By this method a mixture of two products is formed and these were separable by column chromatography. The undesired by-product is eluted off the column first; further elution gives the desired acetal-ester. It is a yellow oil at room temperature and appears to have stability properties similar to GLA, stable in air at room temperature for short periods (days) but is best stored long term in a cool place under nitrogen.ExperimentalOxalyl chloride (2.6 ml, 3.78 g, 30 mmol, 1.5 equiv) was added to a solution of gamma- linolenic acid (GLA, 5.56 g, 20 mmol. 1.0 equiv) in dichloromethane (DCM, 40 ml). The resulting solution was stirred under N2 at room temperature overnight and then concentrated in vacuo. The residual oily gamma-linolenoyl chloride was added dropwise EPO <DP n="17"/>over 10 min to a stirred solution of <strong>[4740-78-7]glycerol formal</strong> (2.50 g, 24 mmol, 1.2 equiv) in DCM (40 ml) containing pyridine (10 ml, 9.78 g, 0.12 mol, 6 equiv) at 5 0C. The reaction mixture was stirred at room temperature for 2h, the precipitated pyridine hydrochloride filtered off, and the filtrate washed with water (2 x ). After drying over MgSO4 the solvent was removed in vacuo to give a light tan oil (6.5 g). This material was chromatographed on silica (60 g). Elution with hexane-ether (94:6) gave 5.2 g of an oil consisting of two components (TLC, HPLC). These were separated on a second silica column (6Og). Elution with hexane-ether (98:2 then 95:5) gave 4-(gamma- linolenoyloxymethyl)- 1,3-dioxolane as a yellow oil (1.2 g, 98% by HPLC). deltaH (500 MHz, CDCl3) 0.89 (3H, t, J= 7.0 Hz, CH3), 1.24-1.45 (8H, complex m, 4 x CH2), 1.65 (2H, p, J= 7.5 Hz5 CH2-C-CO), 2.08 (4H1 m, 2 x CH2C=C), 2.35 (2H3 1, J = 7.5 Hz, CH2CO), 2.80 (4H, t, J = 6.0 Hz, 2 x C=CCH2C=C), 3.67 (IH, m, OCITLambdaHB), 3.97 (IH, m, OCHAi2s), 4.14 (2H5 m, OCHAHB), 4.26 (IH, p, J= 3.5 Hz5 CHO)5 4.89 and 5.02 (2H, 2 x s, OCH2O)5 5.36 (6H, m, 3 x CH=CH). deltac (126.8 MHz, CDCl3) 14.09 (CH3), 22.60, 24.51, 25.65, 26.85, 27.23, 29.16, 29.34, 31.53, 33.97, 63.93 (CH2O), 66.72 (CH2O), 73.31 (CHO)5 95.44 (OCO), [127.60, 128.04, 128.32, 128.41, 129.50, 130.41, olefmic C], 173.26 (carbonyl).Further elution gave 5-(gamma-linolenoyloxy)-l,3-dioxan as a yellow oil (1.6 g, 97.8%) by HPLC). deltaH (500 MHz, CDCl3) 0.89 (3H, t, J= 7.0 Hz, CH3), 1.24-1.46 (8H, complex m, 4 x CH2), 1.67 (2H5 p, J = 7.5 Hz, CH2-C-CO)5 2.05 (4H5 m, 2 x CH2C=C), 2.40 (2H, t, J = 7.5 Hz, CH2CO), 2.81 (4H, t, J = 6.0 Hz, 2 x C=CCH2C=C)5 3.91 (2H5 m, OCH2), 3.99 (2H5 m, OCH2), 4.73 (IH5 p, J= 3.5 Hz5 CHO)5 4.80 (IH, d, J = 6.0 Hz, OCHAHBO), 4.93 (IH, d, J = 6.0 Hz, OCHANo.*O), 5.37 (6H5 m, 3 x CH=CH). deltac (126.8 MHz5 CDCl3) 14.08 (CH3), 22.59, 24.53, 25.65, 26.86, 27.22, 29.06, 29.34, 31.52, 34.12, 65.54 (CHO), 68.55 (CH2O), 93.66 (OCO), [127.60, 128.05, 128.32, 128.42, 129.51, 130.42, olefmic C]5 173.12 (carbonyl). Some fractions containing both compounds were obtained during the chromatography and these could be recycled if necessary to give more material. The reaction scheme for this synthesis is shown in the figures below. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping