Alternatived Products of [ 53896-49-4 ]
Product Details of [ 53896-49-4 ]
| CAS No. : | 53896-49-4 |
MDL No. : | MFCD09881239 |
| Formula : |
C5H3N3
|
Boiling Point : |
No data available |
| Linear Structure Formula : | - |
InChI Key : | PJESVVYWPFAJCS-UHFFFAOYSA-N |
| M.W : |
105.10
|
Pubchem ID : | 13642940 |
| Synonyms : |
|
Application In Synthesis of [ 53896-49-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 53896-49-4 ]
- 1
-
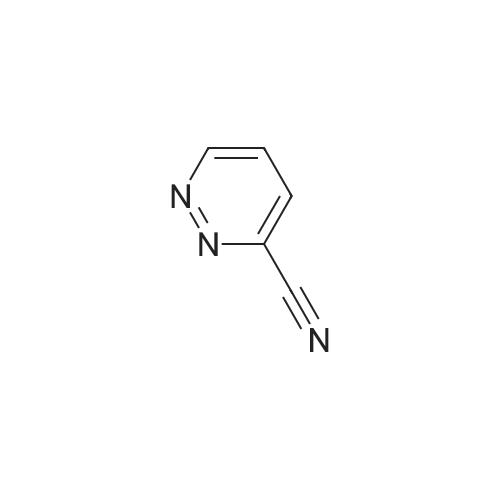 [ 53896-49-4 ]
[ 53896-49-4 ]

-
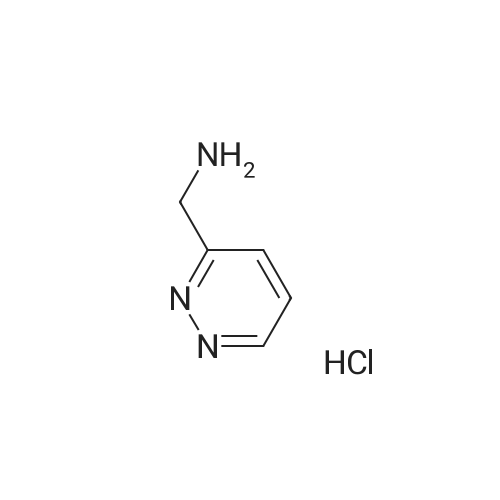 [ 1228788-25-7 ]
[ 1228788-25-7 ]
| Yield | Reaction Conditions | Operation in experiment |
|
With hydrogenchloride; hydrogen;palladium on activated charcoal; In methanol; water; under 2068.65 Torr; for 2.0h; |
Step C; To a solution of the intermediate from step B (5.96 g, 56.7 mmol) in MeOH (35 ml) was added 6N HC1 (20.89 ml, 125 mmol) followed by Pd/C (0.905 g, 8.51 mmol). The reaction mixture was kept on Parr shaker for 2 hours at 40 psi. The reaction mixture was filtered through celite and washed with 600 mL of MeOH and the filtrate concentrated. The residue was azeotroped several times with toluene A dark brown solid obtained. LC-MS: m/z=l 10 (M+l); rt-0.36 (Method A). |
|
With hydrogenchloride; hydrogen;palladium on activated charcoal; In methanol; water; under 2828.7 Torr; for 2.0h;Inert atmosphere; |
To a solution of the intermediate from Step B (5.96 g, 56.7 mmol) in MeOH (35 mL) was added 6N HC1 (20.89 mL, 125 mmol) followed by Pd/C (0.905 g, 8.51 mmol). The reaction mixture was kept on Parr shaker for 2 hours at 40 psig hydrogen. The reaction mixture was filtered through Celite (diatomaceous earth)and washed with 600 mL of MeOH and the filtrate concentrated. The residue was azeotroped several times with toluene. A dark brown solid was obtained, m/z = 110 (M+H). |
|
With hydrogenchloride; palladium on activated charcoal; hydrogen; In methanol; water; under 2068.65 Torr; for 2.0h; |
To a solution of the intermediate from Step B (5.96 g, 56.7 mmol) in MeOH (35 mL) was added 6N HCl (20.89 mL, 125 mmol) followed by Pd/C (0.905 g, 8.51 mmol). The reaction mixture was kept on Parr shaker for 2 hours at 40 psi hydrogen. The reaction mixture was filtered through celite and washed with 600 mL of MeOH and the filtrate concentrated. The residue was azeotroped several times with toluene. A dark brown solid was obtained. LC1 rt = 0.36 min, m/z = 110 (M+H). |
|
With hydrogenchloride; palladium on activated charcoal; hydrogen; In methanol; water; under 2828.7 Torr; for 2.0h; |
To a solution of pyridazine-3-carbonitrile (500 mg, 4.7 mmol) in MeOH (10 mL), was added HCl 6N (2 mL, 12 mmol) followed by Pd/C (50 mg). The reaction mixture was kept on a Parr shaker for 2 hours at 40 psig hydrogen. The reaction mixture was filtered through Celite (diatomaceous earth), washed with 100 mL of MeOH, and the filtrate was concentrated. The residue was azeotroped several times with toluene to give pyridazin-3- ylmethanamine hydrochloride as a dark brown solid (crude 500 mg, quantitative yield). LC- MS (ESI): m/z (M+H) = 110.15. |

- 2
-
 [ 53896-49-4 ]
[ 53896-49-4 ]

-
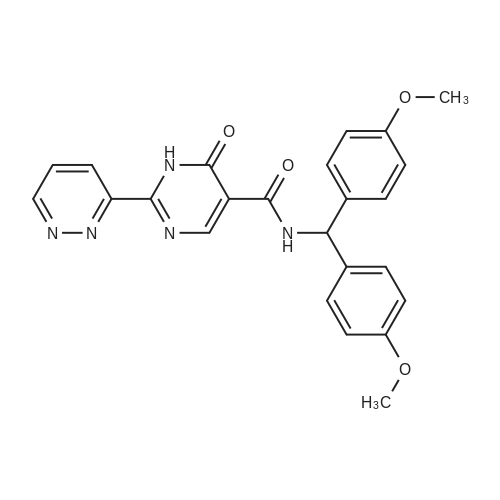 [ 1187990-87-9 ]
[ 1187990-87-9 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping







