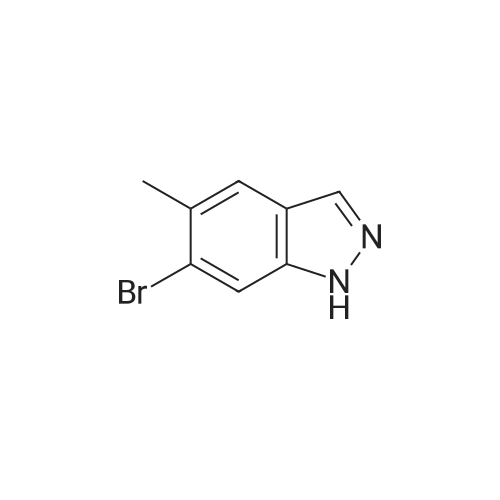
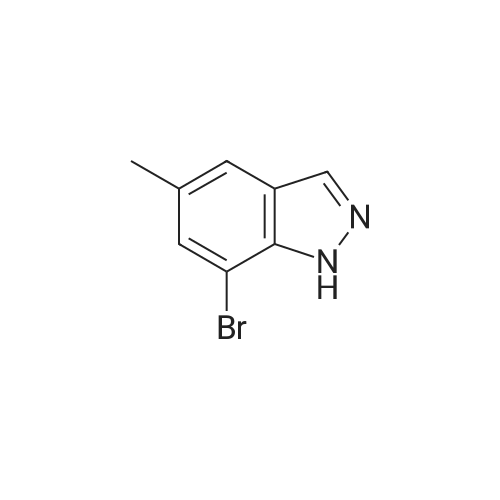
| 36%; 52% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium hydride; In tetrahydrofuran; at 20℃; for 3h; |
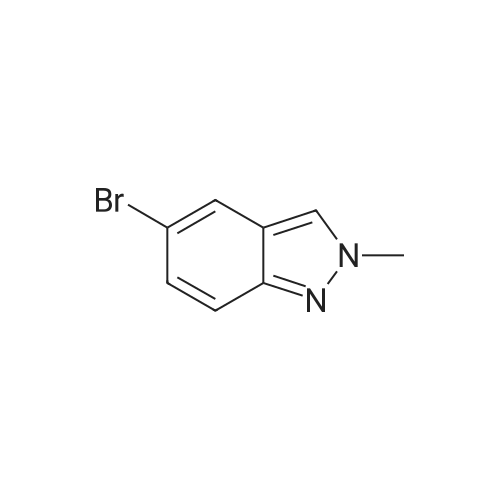
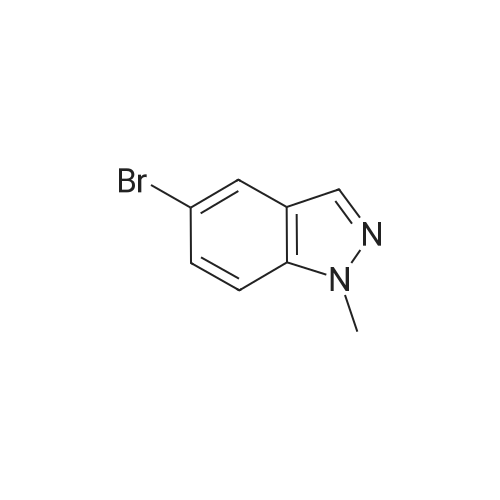
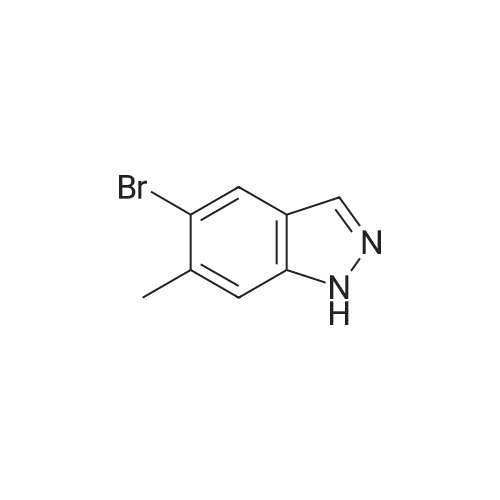
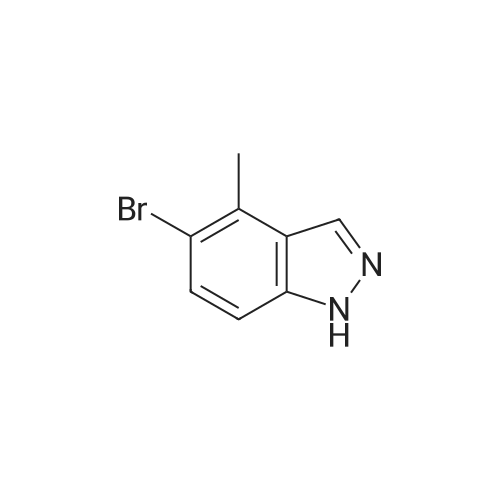
The A14-1 (590mg, 2.99mmol) was dissolved in tetrahydrofuran (10mL), was added NaH in the case of an ice bath with stirring (180mg80%, 6.00mmol),After stirring for 30 minutes was added CH3I (468mg, 3.30mmol), stirring was continued for 3 hours, raised to room temperature, water (20 mL), ethyl acetate (20mL × 3)Extraction, the organic phase was washed with saturated brine (50mL × 3), dried over anhydrous sodium sulfate, and spin dry solvent, the residue was purified by column chromatography (petroleum ether: ethyl acetate= 10:1-3:1) to give A14-2 (230mg, 36%) and A14-3 (330mg, 52%).A14-2: off-white solid. 1HNMR (400MHz, DMSO) delta8.33 (s, 1H), 7.96-7.95 (m, 1H), 7.57 (d, J = 9.2Hz,1H), 7.31-7.28 (m, 1H), 4.16 (s, 3H).A14-3: white solid. |
| 29%; 42.5% |
|
5-Bromo-1-methyl-1 H-indazole (ii) and 5-Bromo-2-methyl-2H-indazole (iii)To a solution of 5-bromo-1 H-indazole (0.19 g, 0.94 mmol) in 3 mL THF at 0 0C was added NaH (0.04 g, 1.03 mmol). The reaction solution was stirred at this temperature for 1 hour before methyl iodide (0.09 mL, 1.41 mmol) was added at 0 0C. The reaction was allowed to warm to room temperature slowly and stirred for 2 hours, quenched with water and concentrated in vacuo. The residue was diluted with water and extracted with DCM twice. The organic layers were combined, dried over Na2SO4 and concentrated. The crude product was purified by flash chromatography (hexane:EtOAc = 10:1 ) to give the title compound ii (88.7 mg, 42.5 %) and iii (60.9 mg, 29 %) as two white solids, ii: 1H-NMR (400MHz, DMSO- Cf6) delta ppm 8.02 (d, 1 H), 7.99 (d, 1 H), 7.64 (d, 1 H), 7.50 (dd, 1 H), 4.04 (s, 3H). LCMS(method A): [MH]+ = 21 1/213, tR = 5.19 min. iii: 1H-NMR (400MHz, DMSO-Cf6) delta ppm 8.33 (s, 1 H), 7.95 (d, 1 H), 7.57 (d, 1 H), 7.30 (dd, 1 H), 4.16 (s, 3H). LCMS (method A): [MH]+ = 21 1/213, tR = 4.95 min. |
| 29%; 42.5% |
|
To a solution of 5-bromo-1 H-indazole (0.19 g, 0.94 mmol) in 3 ml_ of THF at 0 QC was added NaH (0.04 g, 1 .03 mmol). The reaction mixture was stirred at this temperature for 1 h before the addition of methyl iodide (0.09 ml_, 1 .41 mmol) at 0 QC. The reaction was allowed to warm to rt slowly and stirred for 2 h, quenched with water and concentrated in vacuo. The residue was diluted with water and extracted with DCM twice. The organic layers were combined, dried over Na2SO4 and concentrated. The crude products were purified by flash chromatography (hexane:EtOAc = 10:1 ) to give the title compound ii (88.7 mg, 42.5 %) and iii (60.9 mg, 29 %) as white solid, ii: 1H-NMR (400MHz, DMSO-d6) Dppm 8.02 (d, 1 H), 7.99 (d, 1 H), 7.64 (d, 1 H), 7.50 (dd, 1 H), 4.04 (s, 3H). LCMS (method A): [MH]+ = 21 1/213, tR = 5.19 min. iii: 1H-NMR (400MHz, DMSO-Cf6) deltappm 8.33 (s, 1 H), 7.95 (d, 1 H), 7.57 (d, 1 H),7.30 (dd, 1 H), 4.16 (s, 3H). LCMS (method A): [MH]+ = 21 1/213, tR = 4.95 min. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping