| 78% |
With triethylamine; In dichloromethane; at 0℃; for 1h; |
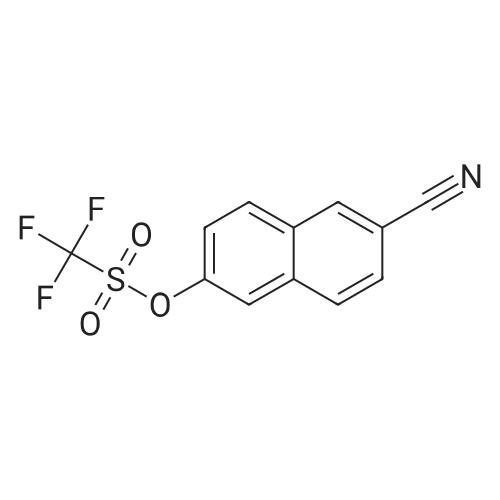
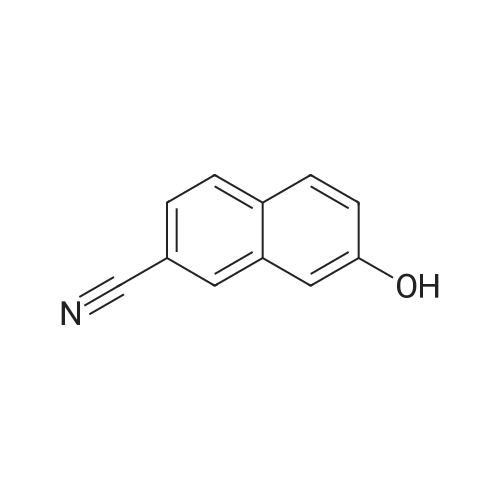
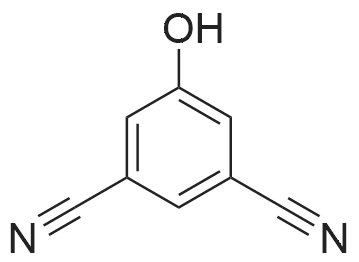
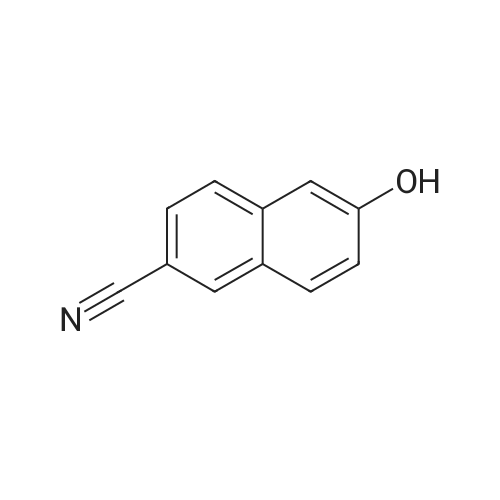
To a stirred solution of 6-hydroxy-2-naphthonitrile N (3.0 g, 17.7 mmol) in DCM (90 mL) were added triethylamine (2.68 g, 26.5 mmol) and triflic anhydride (7.5 g, 26.5 mmol) at 0 C and stirring was continued for an additional 1 h at 0 C. The reaction mixture was partitioned between water and DCM; the organic phase was separated, dried over anhydrous Na2S04, and concentrated under reduced pressure to give crude material. The crude material was purified by column chromatography (Si02, 100-200 mesh) eluting with 3% EtOAc/hexane to afford alcohol O (4.2 g, 13.9 mmol, 78%) as a solid. 1H NMR (200 MHz, CDCb): 5D8.30 (s, 1 H), 8.00 (app t, 2 H), 7.83 (d, 7 = 2.6 Hz, 1 H), 7.74 (dd, 7 = 8.8, 1.8 Hz, 1 H), 7.51 (dd, 7 = 8.8, 2.2 Hz, 1 H). |
| 78% |
With triethylamine; In dichloromethane; at 0℃; for 1h; |
To a stirred solution of 6-hydroxy-2-naphthonitrile N (3.0 g, 17.7 mmol) in DCM (90 mL) were added triethylamine (2.68 g, 26.5 mmol) and triflic anhydride (7.5 g, 26.5 mmol) at 0 C and stirring was continued for an additional 1 h at 0 C. The reaction mixture was partitioned between water and DCM; the organic phase was separated, dried over anhydrous Na2S04, and concentrated under reduced pressure to give crude material. The crude material was purified by column chromatography (Si02, 100-200 mesh) eluting with 3% EtOAc/hexane to afford alcohol O (4.2 g, 13.9 mmol, 78%) as a solid. 1H NMR (200 MHz, CDCb): 5D8.30 (s, 1 H), 8.00 (app t, 2 H), 7.83 (d, 7 = 2.6 Hz, 1 H), 7.74 (dd, 7 = 8.8, 1.8 Hz, 1 H), 7.51 (dd, 7 = 8.8, 2.2 Hz, 1 H). |
|
With triethylamine; In ethanol; dichloromethane; |
EXAMPLE 28B 6-(Trifluoromethanesulfonyloxy)-2-naphthalenecarbonitrile A solution of Example 28A (14.01 g, 82.8 mmol) and triethylamine (9.2 g, 91.1 mmol) in methylene chloride (40 mL) at 0 C. was treated dropwise with trifluoromethylsulfonic anhydride (28 g, 99.4 mmol), warmed to 25 C. for 48 h, concentrated, redissolved in ethanol (50 mL) and triturated with water to precipitate 8.4 g of the title compound. MS (DCI/NH3) m/e 319 (M+NH4)+. |
|
With triethylamine; In dichloromethane; at 0 - 20℃; for 48h; |
Preparation of 6-cvano-2-naphthalenyl thfluoromethanesulfonateTo a solution of 6-cyano-2-naphthol (1 g, 5.91 mmol) and anhydrous NEt3 (anh.) (1 ml, 7.09 mmol) in CH2CI2 anh. (20 ml) and under an inert atmosphere at 0 0C, triflic anhydride [(CF3SO2)2O, 1.20 ml, 7.09 mmol] was added, dropwise. The reaction was brought to room temperature and was then left to react until there was no starting product observed (48 h). The <n="19"/>reaction mixture was concentrated to dryness and the crude product obtained was suspended in EtOH (5 ml_). Water (5 ml_) was added, the mixture was triturated and the resulting suspension was filtered to give a crude product (1.74 g) as a brownish solid. Purification by column chromatography (SiO2, CH2CI2: cyclohexane, 6/4) yielded the compound of the title (1.56 g, 88%) in the form of a white solid IR (KBr) 3058, 2240, 1809, 1630, 1604, 1425, 1152, 964, 932. 1H NMR (400 MHz, CDCI3) 8.30 (d, J = 0.4 Hz, 1 H), 8.03 (d, J = 8.8 Hz, 1 H), 7.99 (d, J = 8.4 Hz, 1 H), 7.83 (d, J = 2.4 Hz, 1 H), 7.73 (dd, J = 8.8 and 1.4 Hz, 1 H) and 7.52 (dd, J = 8.8 and 2.4 Hz, 1 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping