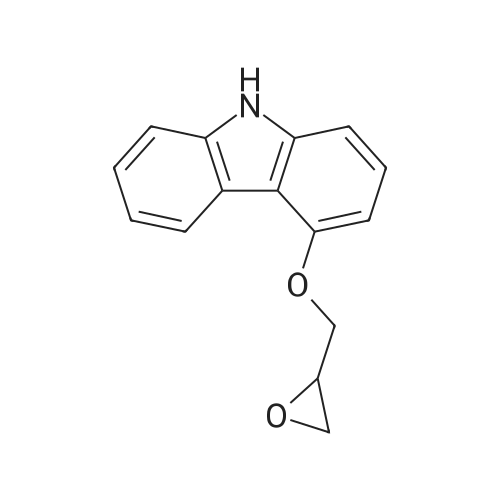
| 65% |
With potassium carbonate; In acetonitrile; for 24h;Reflux; |
The 4 - hydroxy-carbazole (1g, 5.5mmol) for 20 ml acetonitrile dissolved, add catalyst potassium carbonate (1.52g, 11mmol) and epichlorohydrin (5.0 ml, 6 . 4mmol), heating reflux reaction to the raw reaction is complete, the reaction time is about 24h. Evaporate the solvent under reduced pressure, the residue for 50 ml ethyl acetate to dissolve the, water washing 3 times, each 20 ml. The organic layer is separated, and 20 ml saturated salt water washing 1 time, then adding anhydrous sodium sulfate drying, reducing pressure and solvent, it is crude. Purification of the crude product by column chromatography (dichloromethane: petroleum ether=1:1), to obtain a white solid product (0.86g, 65%). |
| 65% |
With potassium carbonate; In acetonitrile; for 24h;Reflux; |
To a suspension of 4-hydroxy carbazole (1 g, 5.5 mmol) andK2CO3 (1.52 g, 11 mmol) in acetonitrile (20 mL), epichlorohydrin(5.0 mL, 6.4 mmol) was added. The reactionmixture was refluxed for 24 h. After completion of thereaction, as indicated by TLC, the acetonitrile in the reactionmixture was removed under reduced pressure. Theobtained residue was dissolved in ethyl acetate (50 mL),organic phase was washed with water (3 × 20 mL), separated,dried with anhydrous Na2SO4, evaporated underreduced pressure, and purified by column chromatographyover silica gel (1:1, dichloromethane/petroleum ether) togive 1 as a white solid (0.86 g, 65%). 1H NMR (400 MHz,CDCl3) delta 8.36 (d, J = 7.9, 0.9 Hz, 1H), 8.10 (s, 1H),7.46-7.39 (m, 2H), 7.35 (t, J = 8.0 Hz, 1H), 7.27 (dd, J =5.8, 2.2 Hz, 1H), 4.49 (dd, J = 11.0, 3.3 Hz, 1H), 4.30 (dd,J = 11.0, 5.4 Hz, 1H), 3.59 (dddd, J = 5.6, 4.1, 3.4, 2.6 Hz,1H), 3.03 (dd, J = 5.0, 4.1 Hz, 1H), 2.92 (dd, J = 5.0, 2.6Hz, 1H). 13C NMR (100 MHz, CDCl3) delta 155.02, 141.01,138.76, 126.62, 125.14, 123.24, 122.55, 119.78, 112.92,110.00, 104.09, 101.39, 68.84, 50.40, 44.92. MS ESI:239.1. |
| 64% |
With sodium hydroxide;tetrabutylammomium bromide; In water; at 30℃; for 2 - 3h; |
Step 2: Preparation of 2,3-Epoxypropoxy carbazole; 4-Hydroxy carbazole (60g) obtained in step 1 was charged into a reactor containing water (90ml). Epichlorohydrin (64.2g) and tetrabutyl ammonium bromide (6.3g) were then charged into the reactor under stirring. 50% Sodium hydroxide solution (78g) was added slowly in 2 - 3 hours into the reaction mass at about 30C. After completion of reaction, ethyl acetate (315ml) and potable water (315ml) were charged into the reaction mass. The lower aqueous layer was separated and discarded. The ethyl acetate layer was washed with potable water to obtain neutral pH and dried over anhydrous sodium sulphate. The ethyl acetate is distilled out under vacuum below 55C to about 100ml. The reaction mass was chilled to 0- 5C and filtered and dried at 50-60C to give the title compound (50g) (64%). |
| 64% |
With potassium carbonate; In acetone; for 24h;Reflux; |
To a suspension of 4-hydroxy carbazole (5 g, 0.027 mol) and K2CO3 (7.54 g, 0.054 mol) in acetone (80 mL), epichlorohydrin (2.50 mL, 0.032 mol) was added. The reaction mixture was refluxed for 24 h. After completion of the reaction, as indicated by TLC, the acetone in the reaction mixture was removed under reduced pressure. The obtained residue was dissolved in ethyl acetate (75 mL), organic phase was washed with water (3 x 15 mL), separated, dried with Na2SO4, evaporated under reduced pressure and purified by column chromatography over silica gel (25:75, ethyl acetate/hexane) to afford 8 as solid compound. Yield 64%; mp 167-169 C; 1H NMR (300 MHz, CDCl3): delta 2.81-2.88 (dd, J = 2.45, 2.64 Hz, 1Ha, CH2), 2.90-2.97 (dd, J = 4.34, 4.91 Hz, 1Hb, CH2), 3.50-3.57 (m, 1Hc, CH), 4.04-4.14 (dd, J = 6.23 Hz, 1He, CH2), 4.52-4.59 (dd, J = 2.26 Hz, 1Hd, CH2), 6.70 (d, J = 7.93 Hz, 1H, Ar-H), 7.05-7.20 (m, 2H, Ar-H), 7.25-7.39 (m, 2H, Ar-H), 7.46 (d, J = 7.93 Hz, 1H, Ar-H), 8.17 (d, J = 7.14 Hz, 1H, Ar-H), 11.27 (s, 1H, NH); ESI MS: m/z = 239 [M+]. |
| 9.8 g (72%) |
With sodium hydroxide; In water; dimethyl sulfoxide; |
Example 14 4-Oxiranylmethoxy-9H-carbazole 10.4 g of 4-hydroxy-carbazole (57 mmol) were dissolved in 31.1 ml of DMSO. 6.9 ml of epichlorohydrin (88 mmol) were added and next 57 ml of a 1 N sodium hydroxide solution. The mixture was stirred for 8 h at 40 C. It was cooled to 20 C. adn 130 ml of water were added. The product was filtered under suction, and washed with 3*30 ml water. The crude material was recrystallized from isopropanol. The substance was dried at 60 C. for 12 h. Yield: 9.8 g (72%), m.p. 128-132 C. |
|
With potassium carbonate; In ipa; at 75 - 85℃; for 6h; |
IPA (70ml) was added to a mixture of 4-etaydroxy carbazole (0.05458 moles, 10 g) and epichlorohydrin (0.10916 moles, 8.5 ml). Potassium carbonate (0.13645 moles, 18.85 g) was added to the above reaction mixture and stirred. Subsequently the reaction mixture was heated to 75-85 0C. After 6 hours when the reaction was complete, the reaction mixture was slowly cooled to room temperature and filtered. The filtrate was reduced to 80% of its volume, and stirred at room temperature, when a solid was formed which was filtered to give the product. |
|
With potassium carbonate; In isopropyl alcohol; at 75 - 85℃; for 6h; |
Step 1: Preparation of 4-(Oxiran-2-ylmethoxy)-9H-carbazole IPA (70 ml) was added to a mixture of 4-Hydroxy carbazole (0.05458 moles, 10 g) and epichlorohydrin (0.10916 moles, 8.5 ml). Potassium carbonate (0.13645 moles, 18.85 g) was added to the above reaction mixture and stirred. Subsequently the reaction mixture was heated to 75-85 C. After 6 hours when the reaction was complete, the reaction mixture was slowly cooled to room temperature and filtered. The filtrate was reduced to 80% of its volume, and stirred at room temperature, when a solid was formed which was filtered to give the product. |
|
|
General procedure: Commercially unavailable 2-(aryloxymethyl)oxiranes were synthesized by following procedure as previously reported. To a solution of corresponding phenols in dimethyl sulfoxide (DMSO) at room temperature,NaOH aqueous solution (1 M, 1.2 equiv) was added, the volume ratio of solvent is DMSO:H2O = 2:1, the mixture was stirred for10 min and epichlorohydrin (1.5 equiv) was added dropwise within10 min, the reaction mixture was allowed to stirred for further 2 h.AcOEt was added to the mixture, which was washed by brine for 3times. The solvents were evaporated under vacuum, and the crude residuewas purified by column chromatography on silica gel. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping