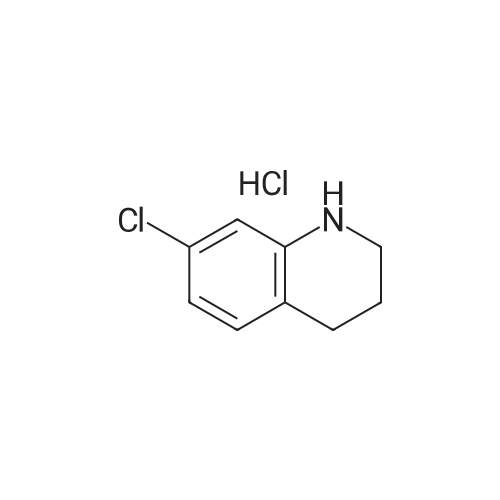
| 86% |
|
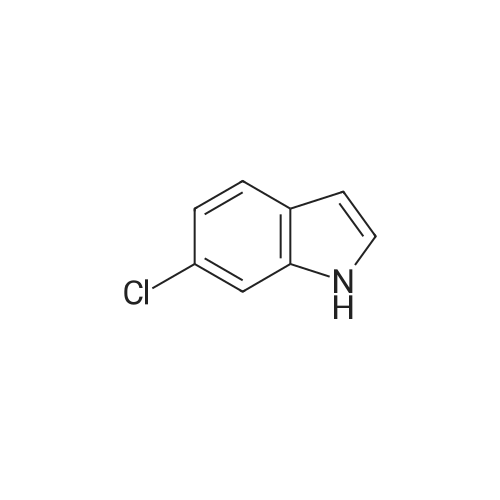
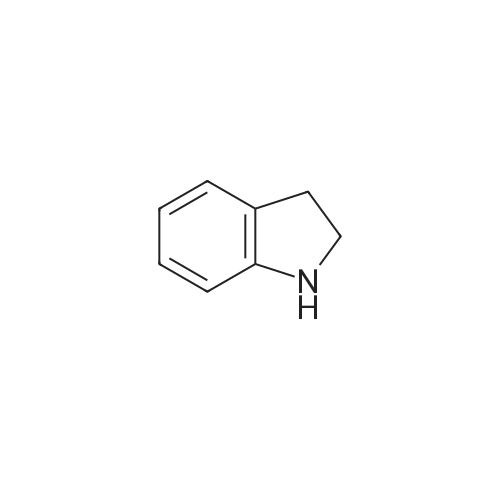
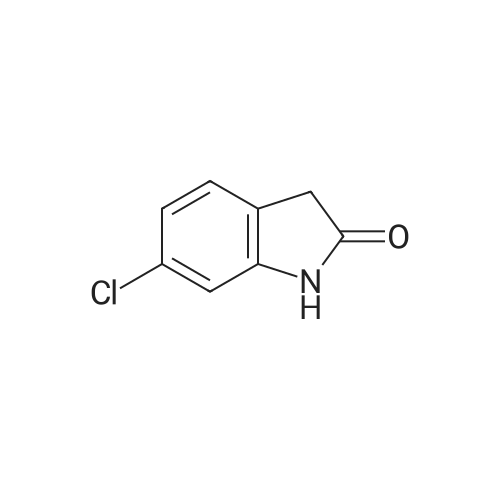
Preparation 30: 6-Chloro-2,3-dihydro-1 H-indole6-Chloroindole (1.0 g, 6.6 mmol) was dissolved in a solution of borane in THF (1 M, 9.83 mmol) at 0 C and stirred for 30 min. TFA (9.83 mL) was added dropwise and the solution stirred at 0 C for 30 min. 6 M aqueous NaOH was added until the solution was basic (pH 1 1 ). The aqueous solution was extracted with DCM (3 x 25 mL), dried over sodium sulfate, filtered and concentrated to give the title compound (864 mg, 86%) as a yellow oil. 1H NMR (Me-d3-OD): 6.99 (1 H, d), 6.64-6.55 (2H, m), 3.50 (2H, t), 2.95 (2H, t). |
| 72% |
With sodium cyanoborohydride; for 22h; |
In a 250 mL round bottom flask, 12.4 grams of sodium cyanoborohydride (198 mmol, 2 eq. ) were added potion-wise over 5 minutes to a solution of 15 grams (98.9 mmol) of 6-chloroindole. After stirring for 22 hours, the mixture had become a brown solution and analysis by [HPLC] (MRH 1 method) revealed no starting material remaining and a mixture of two product peaks. The mixture was diluted with 100 [ML] of water, then made basic [WITH-200] mL of 6N sodium hydroxide. The desired product was extracted into 3 X 400 mL of methylene chloride. The extracts were then dried over anhydrous magnesium sulfate and evaporated in vacuo leaving a cloudy oil. The crude product was chromatographed over a plug of silica in 100 % methylene chloride giving a mixed fraction [(RF=] 0.9 and 0.7), a pure product fraction [(RF= 0. 7),] and a baseline fraction (Rf = 0.0-0. 2). The pure fraction was evaporated to dryness in vacuo to yield a clear, colorless oil weighing 10.90 grams (72 %). It was stored at [4C] and saved for future [USE. 1H] NMR (300 MHz, DMSO-d6) 8 6.95 (d, J= 5 Hz, 1 H), 6.46 (d, [J=] 5 Hz, 2 H), 3.43 (t, [J=] 6,2 H), 2.86 (t, [J=] 6,2 H). |
|
With sodium cyanoborohydride; In acetic acid; at 20℃; for 1h; |
[0278] 6-Chloroindole (1 g, 6.6 mmol) in glacial acetic acid (10 mL) was treated with sodium cyanoborohydride (829 mg, 13.2 mmol) portionwise at room temperature with stirring. After 1 hour, the reaction was diluted with water (25 mL) and basified with 40% sodium hydroxide with cooling. The mixture was then extracted with dichloromethane (3×50 mL), dried and concentrated to give 1 g of 6-chloroindoline. It was used in the next step without further purification. 1HNMR (300 MHz, dimethylsulfoxide-d6) delta 6.95 (d, 1H), 6.46 (dd, 1H), 6.43 (d, 1H), 5.74 (br s, 1H, NH), 3.42 (t, 2H, CH2), 2.85 (t, 2H, CH2). MS m/z 349 [M+1]. |
|
|
EXAMPLE 208a Preparation of intermediate 6-chloro-2,3-dihydro-1H-indole Sodium borohydride (2.0 g, 53 mmol) (Aldrich) was added in small portions to a mixture of 6-chloro-1H-indole (1.0 g, 6.6 mmol) (Aldrich) in TFA (10 mL), which was cooled in ice-water bath, at such a rate that gas evolution was not too vigorous. When the addition was complete, the mixture was allowed to warm to room temperature and stirred overnight. The resulting mixture was concentrated in vacuo and the residue was dissolved in DCM. The organic layer was washed with Na2CO3 solution, dried with Na2SO4, and concentrated to give 0.6 g crude 6-chloro-2,3-dihydro-1H-indole. MS: [M+H]+=154 |
| 16%Chromat. |
With 6C53H32O8(4-)*13Zr(4+)*18O(2-)*8Co(2+)*8Cl(1-); hydrogen; sodium triethylborohydride; In toluene; at 80℃; under 30003.0 Torr; for 72h; |
General procedure: At a 0.5 mol % Co loading, Zr-MTBC-CoH catalyzed hydrogenation of indole in toluene at 80 C. to afford a mixture of indoline and 4,5,6,7-tetrahydroindole. Indoline was obtained in 84% isolated yield after preparative TLC. See first entry, Table 19, below. Hydrogenation of 3-methyl-indole gave 3-methyl-indoline and 3-methyl-4,5,6,7-tetrahydroindole in 46:54 ratio, which indicates that reduction of the phenyl ring is also possible. Hydrogenation of quinolines in toluene at 80 C. gave a mixture of two products, 1,2,3,4-tetrahydroquinoline and 5,6,7,8-tetrahydro-quinoline in a 1:1 ratio. Under identical reaction conditions, the selectivity appears dependent on the substitution of the phenyl ring. Electron-donating substituents at the 6-position of the quinolines favor the hydrogenation of the phenyl ring. For example, the 6-methylquinoline, 6-methoxyquinoline and 2,6-dimethylquinoline were hydrogenated to give 6-methyl-5,6,7,8-tetrahydro-quinoline, 6-methoxy-5,6,7,8-tetrahydro-quinoline and 2,6-dimethyl-5,6,7,8-tetrahydro-quinoline, respectively, as the major products. See Table 19. In contrast, strong electron-withdrawing substituents seem to disfavor the reduction of the phenyl ring. The hydrogenation of 2-methyl-6-fluoro-quinoline afforded 2-methyl-6-fluoro-1,2,3,4,-tetrahydro-quinoline exclusively in 72% yield. See second to last entry, Table 19. Zr-MTBC-CoH was also an active catalyst for hydrogenation of benzofuran. At a 0.2 mol % Co loading, benzofuran was completely hydrogenated to 2,3-dihydrobenzofuran in qualitative yield. See next to last entry, Table 19. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping