| 61% |
|
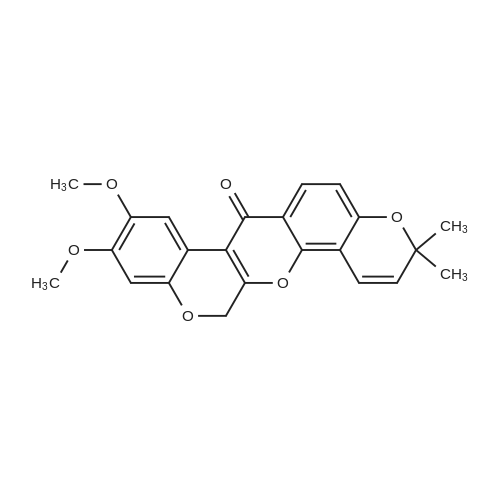
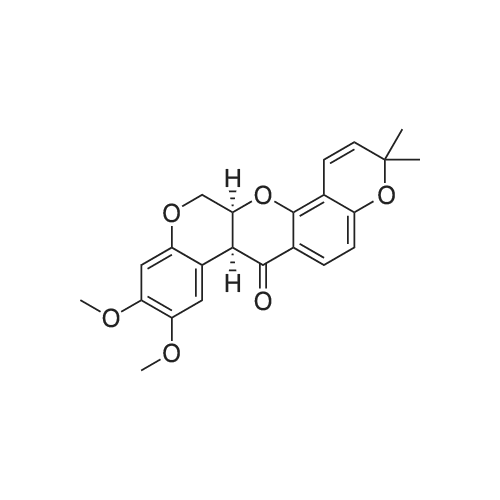
Comparative Example 1: Preparation of (7aS,13aS)-9,10-Dimethoxy-3,3-dimethyl-13,13a-dihydro-3H-chromeno[3,4-b]pyrano[2,3-h]chromen-7(7aH)-one (Deguelin) Phenylselenyl chloride (68 mg, 0.35 mmol) was added to anhydrous CH2Cl2 solution (4.0 mL) containing the compound 8 (128 mg, 0.32 mmol) prepared in Example 1 at -30°C under argon atmosphere, followed by stirring for 10 minutes with maintaining the temperature at -30°C. The temperature was raised to room temperature with stirring for 2 hours, and then additional stirring was performed for 1 more hour. The solvent was eliminated from the reaction mixture under reduced pressure and the obtained residue was dissolved in THF (4.0 mL), to which hydrogen peroxide (30percent in water, 0.06 mL) was added 0°C. The reaction mixture was stirred until the temperature of the mixture reached to room temperature, during which the reaction was monitored with TLC. EtOAc (8.0 mL) and water (4.0 mL) were added thereto. The organic layer was separated, washed with 5percent NaHCO3 aqueous solution and brine, dried over MgSO4, filtered, and then concentrated. The obtained non-purified residue was purified by flash column chromatography (EtOAc:n-hexane=1:2) to give deguelin as a light-yellow solid (yield: 61percent, 78 mg). 1H-NMR (CDCl3, 400 MHz) delta 7.72 (d, 1H, J = 8.7 Hz), 6.77 (s, 1H), 6.62 (d, 1H, J = 10.0 Hz), 6.43 (s, 1H), 6.43 (d, 1H, J = 8.7 Hz), 5.53 (d, 1H, J = 10.0 Hz), 4.89 (m, 1H), 4.61 (dd, 1H, J = 12.0, 3.1 Hz), 4.17 (d, 1H, J = 12.0 Hz), 3.82 (d, 1H, J = 4.1 Hz), 3.78 (s, 3H), 3.75 (s, 3H), 1.43 (s, 3H), 1.36 (s, 3 H) ; 13C-NMR (CDCl3, 100 MHz) delta 189.2, 160.0, 156.9, 149.4, 147.4, 143.8, 128.6, 128.5, 115.7, 112.7, 111.4, 110.4, 109.1, 104.7, 100.9, 77.6, 72.4, 66.2, 56.3, 55.8, 44.3, 28.4, 28.1; HRMS (FAB) Calcd for C23H23O6 (M+H+) : 395.1495, Found: 395.1495. |
| 61% |
|
Phenylselenyl chloride (68 mg, 0.35 mmol) was added to anhydrous CH2Cl2 solution (4.0 mL) containing the compound 8 (128 mg, 0.32 mmol) prepared in Example 1 at ?30° C. under argon atmosphere, followed by stirring for 10 minutes with maintaining the temperature at ?30° C. The temperature was raised to room temperature with stirring for 2 hours, and then additional stirring was performed for 1 more hour. The solvent was eliminated from the reaction mixture under reduced pressure and the obtained residue was dissolved in THF (4.0 mL), to which hydrogen peroxide (30percent in water, 0.06 mL) was added 0° C. The reaction mixture was stirred until the temperature of the mixture reached to room temperature, during which the reaction was monitored with TLC. EtOAc (8.0 mL) and water (4.0 mL) were added thereto. The organic layer was separated, washed with 5percent NaHCO3 aqueous solution and brine, dried over MgSO4, filtered, and then concentrated. The obtained non-purified residue was purified by flash column chromatography (EtOAc:n-hexane=1:2) to give deguelin as a light-yellow solid (yield: 61percent, 78 mg). [0584] 1H-NMR (CDCl3, 400 MHz) delta 7.72 (d, 1H, J=8.7 Hz), 6.77 (s, 1H), 6.62 (d, 1H, J=10.0 Hz), 6.43 (s, 1H), 6.43 (d, 1H, J=8.7 Hz), 5.53 (d, 1H, J=10.0 Hz), 4.89 (m, 1H), 4.61 (dd, 1H, J=12.0, 3.1 Hz), 4.17 (d, 1H, J=12.0 Hz), 3.82 (d, 1H, J=4.1 Hz), 3.78 (s, 3H), 3.75 (s, 3H), 1.43 (s, 3H), 1.36 (s, 3H); [0585] 13C-NMR (CDCl3, 100 MHz) delta 189.2, 160.0, 156.9, 149.4, 147.4, 143.8, 128.6, 128.5, 115.7, 112.7, 111.4, 110.4, 109.1, 104.7, 100.9, 77.6, 72.4, 66.2, 56.3, 55.8, 44.3, 28.4, 28.1; [0586] HRMS (FAB) Calcd for C23H23O6 (M+H+): 395.1495. Found: 395.1495. |
|
|
Solid PhSe-Cl (185 mg, 0.972 mmol) is added to a cooled (-30° C.) solution of (-)-rot-2'enonic acid (350 mg, 0.884 mmol) in dichloromethane (10.5 mL). After completion of addition the reaction mixture is allowed to warm to room temperature over 2 h and continues to stir at room temperature for an additional hour. After three hours of total reaction time the reaction mixture is concentrated to yield a yellow oil. The crude material is dissolved in THF (10.5 mL) and cooled to 0° C. Hydrogen peroxide (30percent in water, 0.177 mL) is added. After completion of addition the reaction mixture continues to stir at 0° C. for one hour and then stirs at room temperature overnight. The next day the reaction mixture is diluted with diethyl ether. The organic layer is separated and washed with 5percent NaHCO3 (2.x.), dried over Na2SO4 and concentrated to yield (6aS, 12aS)-deguelin as a yellow amorphous solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping