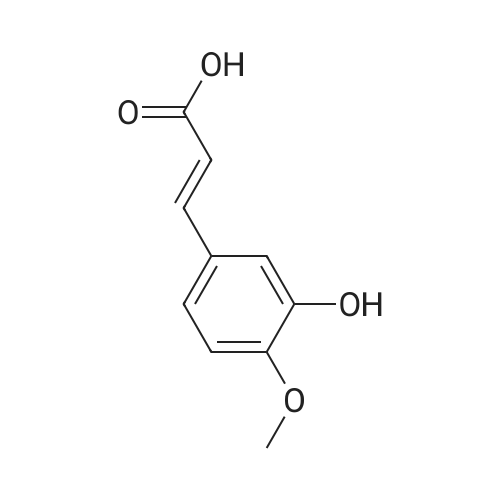
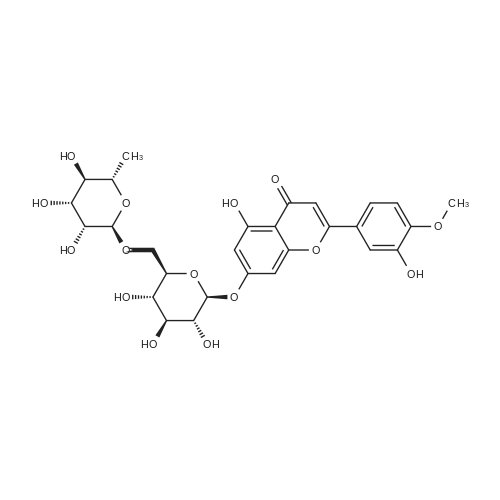
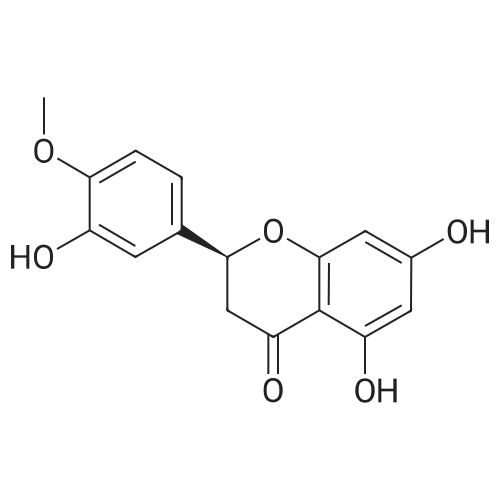
| 85.7% |
With morpholine; iodine; at 40 - 70℃;Large scale; Green chemistry; |
In 20L reaction kettle, add hesperidin 2 kg, iodine 0.92 kg, then add morpholine 12L. Start stirring and elevating temperature. When the temperature rises to 40 deg.C, sample is completely dissolved, At 45 deg.C ± 5 deg.C maintain temperature for 0.5 hours.Then, increase the temperature to 70 deg.C ± 5 deg.C and maintain the temperature and stir for 1.5-3 hours (HPLC monitoring hesperdin completely after transformation as the end point of the reaction). Recovery solvent to reactant into a viscous, adding 12L purified water, stirring, the material after being homogenized, the hydraulic the reaction buffer tank, adding hydrochloric acid adjusting pH=5-7 to crystallization, filtration, filter cake and filtrate, the filtrate pH=2 adjusted by adding hydrochloric acid, slowly adding 30% hydrogen peroxide 0.41 kg, filter, recovery elemental iodine. The filter cake is washed with a large amount of water washing, diosmin mode crude product obtained. Dissolving the purified water is added to the purification tank 12L and 0.39 kg sodium hydroxide, stir until completely dissolved after diosmin mode crude product into the continue to stir until completely dissolved, slowly adding at 20L acetonitrile, the solid is separated out, filtering, takes filters cake (diosmin mode sodium salt purity is of 99.27%), again dissolving the purified water is added to the purification tank 30L, then add and stir the filter cake to be completely dissolved, slowly dilute hydrochloric acid in this case adjusting pH=5-7, a large number of solid precipitation, filtration, the filter cake is washed with a large amount of purified water washing 1 hours, collecting filter cake, is put into the 80 C ± 5 C blast drying, crushing, diosmin mode the finished product to 1.69 kg, the purity is 99.44%, the yield is 85.7%, to the solvent recovery rate 80.1%, iodine recovery rate 89.6%, residual solvent, -related impurities are standard. |
|
With pyridine; iodine; sodium hydroxide; at 95 - 105℃;Product distribution / selectivity; |
100 gm of hesperidin , 700 ml of recovered pyridine, 9.8 gm of sodium hydroxide and 45.6 gm of iodine were charged in 2 liter clean glass assembly . The resulting solution was heated to 95-1050C for 9 - 10 hrs. Reaction was monitored by HPLC. Pyridine was recovered completely by distillation. Charged methanol to the resulting solid, the reaction mass was heated to reflux and filtered at room temperature. The solid obtained was treated with sodium thiosulfate solution and 900 ml, 5% aqueous NaOH solution. pH 2-4 was adjusted with cone, sulfuric acid. Reaction mass was filtered to obtain crude diosmin. Crude diosmin obtained was 80 - 86 gm. Purity was 98.6 %. |
| 13.1 g |
|
Take an amount of 95% hesperidin 20g (previously 90 dried 3-4h), DMSO50ml dissolved by heating, the other to take 3gKBH4, graded by adding, to the reduction of hesperidin naringin type flavan-4- alcohol completely (approximately overnight , UV monitor, under alkaline conditions scans at no absorption peak at 360nm).Slowly adding 5ml of concentrated sulfuric acid (2ml particularly slow first, the solution became light red), mix well (solution becomes light red) was added slowly added anhydrous n-amyl alcohol 120ml, precipitation sink to the bottom, still 30min, poured out positive amyl alcohol solution was added to the precipitate in anhydrous n-amyl alcohol 20ml, stirring rapidly pinching solution, n-amyl alcohol was decanted, treated twice with the law, anhydrous pyridine 40ml, by adding iodine 8.3g, and stir until dissolved, no calcium sulfate 5g, stir, sealed in a water bath at 55 96h after continuous reaction.Activated carbon was added 5.0, add water 500ml, stirring slowly adding phosphoric acid modulation pH5, filtration, the filtrate was adsorbed by 300gD101 macroporous resin, 500ml water to remove pyridine, iodine ions and other impurities, and then 0.1% 0.5% phosphoric acid vitamin C400ml further elution remove residual iodine, pyridine and other impurities, washed with water 300ml, 2000ml eluted with 60% ethanol, recovering ethanol under reduced pressure.After recovery of ethanol was combined with activated charcoal 2g, mixing, filtration, water was recovered under reduced pressure to dryness to obtain Diosmetinidin-7-O-rutinoside, orange powder 13.1 g. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping