| 68.6% |
With phosphorus pentachloride; trichlorophosphate; at 0 - 120℃; for 1.0h; |
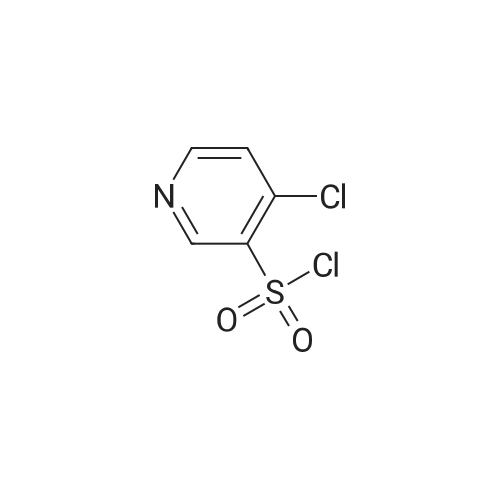
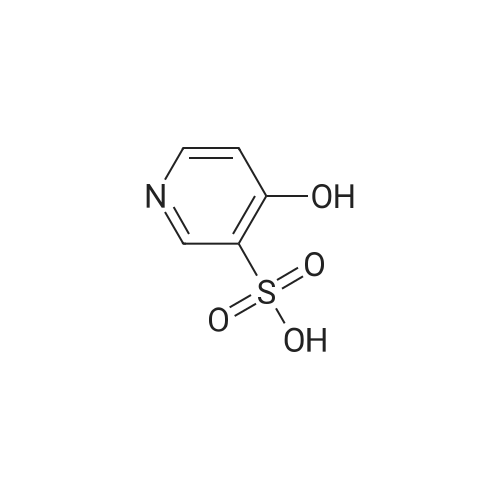
To a suspention of <strong>[51498-37-4]4-hydroxypyridine-3-sulfonic acid</strong> (25 g, 143 mmol) was added PCl5 (104 g, 500 mmol) and POCl3 (26.6 ml, 285 mmol) at 0 C and stirred at 120 C for 1h. The reaction mixture was cool to ambient temperature, the reaction mass was concentrated. The residue was diluted with EtOAc and poured in to ice. Solid NaHCO3 was added and the aqueous phase was extractred with EtOAc.The organic layer was dried over anhydrous Na2SO4 and filtered to afford the title compound (25 g, 98 mmol, 68.6% yield). LCMS m/z 212 (M+H)+, 1.93 min (ret. time). |
|
With phosphorus pentachloride; In chlorobenzene; for 6.0h;Heating / reflux; |
Example 2; 4-Chloro-3-pyridinesulfonamide [4]. "One-pot"procedure:; A mixture of <strong>[51498-37-4]4-hydroxy-3-pyridinesulfonic acid</strong> [5] (50.0 g, 0.29 mol), phosphorus pentachloride (130. 8 g, 0.63 mol) and chlorobenzene (580 g) was stirred under reflux conditions for 6 hours. The mixture of phosphorus oxychloride and chlorobenzene (590-610 g) were distilled off from the mixture at atmospheric pressure. The cooled (25-30 C) residue (-100 mL) was added dropwise to the stirred mixture of 25 % aqueous ammonia (116.5 g, 1.7 mol) and acetone (20 g) for 30-40 minutes maintaining the temperature at 0-5 C. The reaction mixture (pH 11-12) was stirred for 1 hour at the same temperature and for 1 hour at 25 C. Chlorobenzene (50 g) and water (150 g) were added to the stirred mixture at the same temperature. The reaction mixture was concentrated to 250-300 g (pH 7-8) under reduced pressure (20 - 100 mbar) at 30-40 C. The obtained suspension was stirred for 5 hours at 0- 5 C. The precipitated solids were filtered off, triturated with water (2 x 200 g) at 30 C and dried under reduced pressure at 40-50 C (water bath) to a constant weight to give 42.7 g (77.6 %) of 4-chloro-3-pyridinesulfonamide [4] with 99.6 % purity by HPLC, assay 99.9 % by HC104 titration, 0.06 % of water by KF titration and mp 146-148 C (dec. ). |
|
With N-ethyl-N,N-diisopropylamine; trichlorophosphate; In 1,2-dichloro-ethane; at 90℃; for 0.75h; |
4-Chloro-N-(3,4-dimethoxyphenyl)pyridine-3-sulfonamide (IntDI ) A solution of <strong>[51498-37-4]4-hydroxypyridine-3-sulfonic acid</strong> (5.0 g, 28.5 mmol), N,N- di/'sopropylethylamine (9.2 g, 71.3 mmol), phosphorus(V) oxychloride (10.9 g, 71.3 mmol) in 1 ,2-dichloroethane (50 mL) was heated at 90C for 45 min with TLC monitoring (hexane:EtOAc, 1 :1). After cooling, the solution was added to 3,4- dimethoxyaniline (4.8 g, 31.0 mmol), A/./V-di/sopropylethylamine (7.35 g, 57.0 mmol) in 1 ,2-dichloroethane (50 mL) at -10C and the resulting mixture stirred for approximately 2 h at rt with TLC monitoring (hexane:EtOAc, 1 :1). The mixture was then added to saturated aqueous NaHC03 (200 mL), extracted with DCM (3 x 50 mL) and the combined organic layers dried over Na2S04 and concentrated in vacuo. Purification by gradient column chromatography eluting with 0-20% EtOAc in hexane yielded the title compound (2.2 g, 6.69 mmol). Mass spectroscopy: (ESI +ve) 329.2, 331.2 [M+H]+1H NMR: (400 MHz, DMSO) delta 3.65 (s, 3H), 3.65 (s, 3H), 6.60 (dd, J=8.5, 2.4, 1 H), 6.72 (d, J=2.4, 1 H), 6.81 (d, J=8.9, 1 H), 7.78 (d, J=5.2, 1 H), 8.72 (d, J=5.2, 1 H), 8.96 (s, 1 H), 10.52 (br s, 1 H) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping