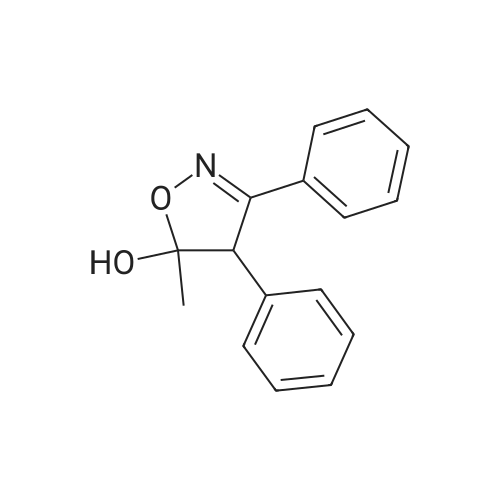
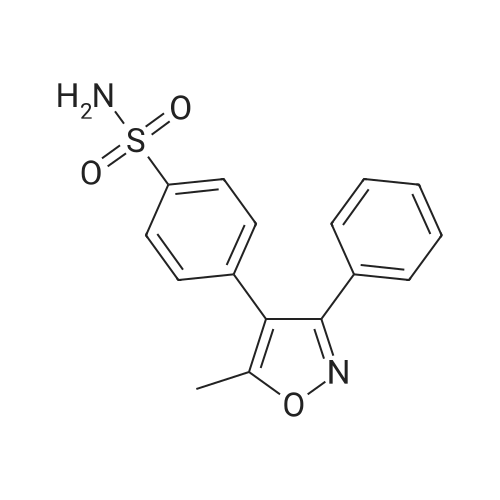
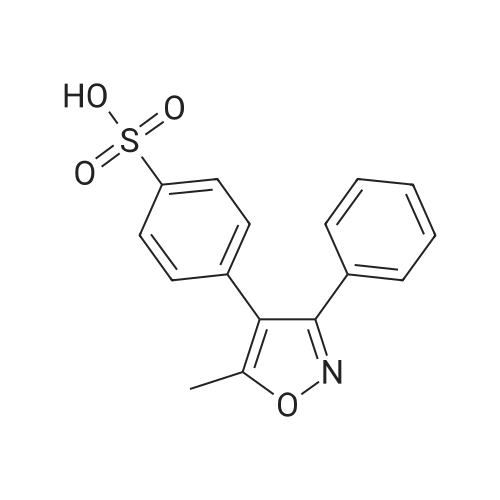
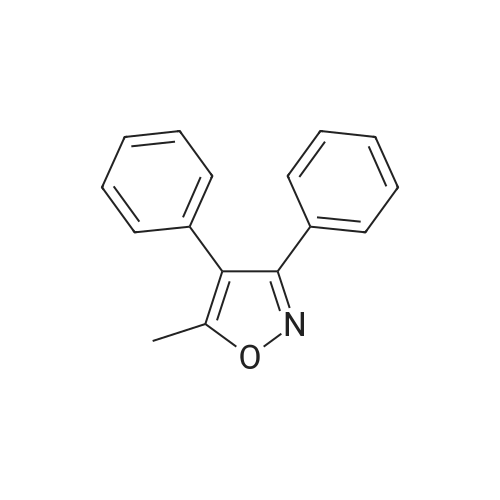
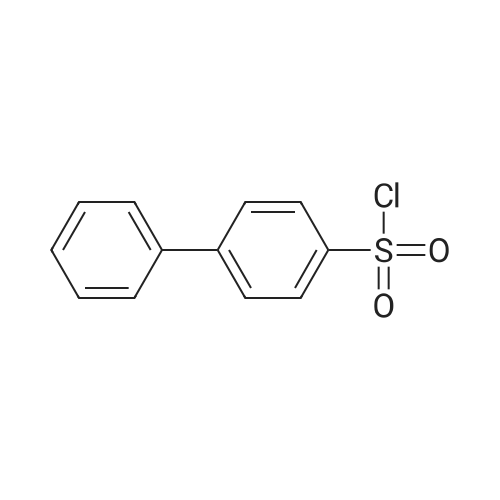
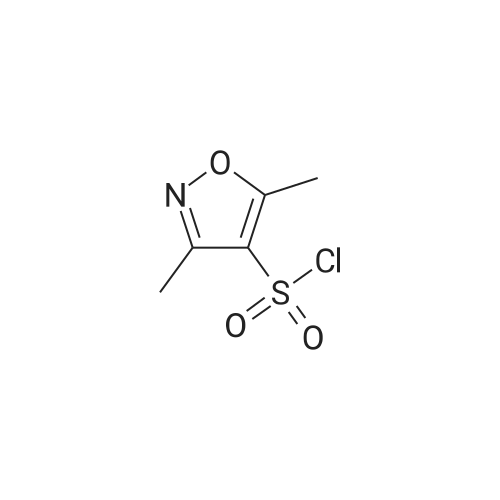
| 77% |
With ammonium hydroxide; In dichloromethane; at 10 - 50℃;Cooling with ice; |
The system was added dropwise to 1200 ml of ice water.Temperature control is about 10 degrees,Liquid separation extraction,The organic phase is washed twice with water.The organic phase is stirred with ammonia (700 ml) at room temperature.The dot plate shows that the reaction is complete,(PE: EA=4:1),The organic phase is removed by a 50 C rotary steaming system.The remaining system was added with 2 L of ethyl acetate.1L of water is stirred until it is dissolved.Liquid separation extraction,The aqueous phase was back extracted with ethyl acetate.Combine the organic phase,Wash with purified water,The organic phase was removed by rotary evaporation to give a crude material.Add 1L of ethanol,Heat to dissolve,Recrystallization,Filtered to give a crude product (350g).The final product 310 g (986.13 mmol) was obtained (HPLC > 99.5%).White solid, yield 77%. |
|
With ammonia; In water monomer; at 20 - 60℃;Product distribution / selectivity; |
EXAMPLE 2 25 ml of 19.5% w/v aqueous ammonia solution were added slowly to a solution of 4-(5-methyl-3-phenyl-4-isoxazolyl) benzene sulfonyl chloride (5 grams) in water (25 ml) at 25-35 C. The reaction mass was heated to 55-60 C. and stirred at this temperature until completion of the reaction. The reaction mass was cooled to about 20-30 C. The solid was filtered and washed with water (30 ml). The compound was suction dried under reduced pressure followed by drying at 25-35 C. under reduced pressure to obtain the desired crystalline Form A of valdecoxib. The yield of the compound was 4.2 grams. |
|
With ammonia; In dichloromethane; water monomer; at 25 - 30℃; for 0.583333 - 0.75h;Product distribution / selectivity; |
EXAMPLE 1; 25 ml of 19.5% w/v aqueous ammonia solution were added slowly to a solution of 4-(5-methyl-3-phenyl-4-isoxazolyl) benzene sulfonyl chloride (5 grams) in dichloromethane (50 ml) at 25-30 C. 0.5 grams of crystalline Form A of valdecoxib (as seeding material) was charged to the reaction mass at 25-30 C. and the reaction mass was stirred for 35-45 minutes. The separated solid was filtered and washed with dichloromethane (5 ml). The compound was suction dried under reduced pressure followed by drying at 35-40 C. under reduced pressure to obtain the desired crystalline Form A of valdecoxib. The yield of the compound was 4 grams and the X-ray diffraction pattern for the product is shown in FIG. 1. |
|
With ammonia; In water monomer; at 0℃; for 1h;Product distribution / selectivity; |
Example 16: 4-f3-Methyl-5-phenyl-4-isoxazolyllbenzenesulfonamide (valdecoxib, 19).; A solution of 5-methyl-3,4-diphenyl isoxazole 18 (250 mg, 1.1 mmol) in chlorosulfonic acid (1 ml) was stirred at 0C for 3 hours. The reaction was cautiously added to concentrated ammonium hydroxide (6 ml) in the cold (0C). The resultant reaction mixture was stirred at 0C for 1 hour. The reaction was cautiously diluted with water and extracted with ethyl acetate. The combined organic layers were dried over sodium sulfate, filtered, and the filtrate concentrated in vacuo to give the crude product. This material was chromatographed on silica gel using 25% ethyl acetate in toluene as the eluent to give the desired sulfonamide as a crystalline solid (110 mg, 40% yield of 19) : mp 85 - 87C. Anal. Calc'd. for C16H14N2O3 S: C, 61.13; H, 4.49; N, 8.91; S, 10.20. Found: C, 60.88; H, 4.61; N, 8.55; S, 10.40. |
|
With ammonia; In dichloromethane; at 0℃; for 1h; |
EXAMPLE 4 4- (5-Methyl-3-phenyl-4-isoxazolyl) benzenesulfonamide (step 3) Charged 400 ml of dichloromethane in a dry 1L four neck round bottom flask, into this 7.8 g of sulfonyl chloride from example 3 (0.02296 mol) was added at room temperature and stirred well. Reaction mass cooled to 0C and ammonia gas was purged for an hour, during this white solid was formed in the reaction mass. After one hour analysed by HPLC showed completion of reaction. Reaction mass allowed to come to room temperature and dichloromethane layer is washed thrice with water (3 x 100 ml). Dried over anhydrous sodium sulfate and filtered. Dichloromethane is distilled to obtain a one fourth of the initial volume and then cooled to 0 to 5C to get the product crystallised. The product is then filtered and washed with pre-cooled dichloromethane (2 x 10 ml) and the product dried under vacuum at 60 to 70C to obtain a crystalline product (5.6 g, HPLC purity = 99. 7%). |
|
With ammonium hydroxide; In dichloromethane; at 20 - 30℃;Large scale; |
The 3.0 kg 5 - methyl - 3, 4 - diphenyl isoxazole added to the 30 L glass reaction flask, then adding 9.9 kg methylene chloride, stirring to dissolve, cooling the solution to - 5 - 0 C, to the reaction solution slowly dropping 15.0 kg chloro sulfonic acid and temperature control ≤ 20 C, after dropping, water bath heating to 35 C insulation reaction 4 - 5 of H, then the reaction liquid cooling to 0 - 5 C.The 15.9 kg methylene chloride and 12.0 kg purified water by adding another 50 L in the reaction bottle, cooling to 0 - 5 C, slowly dropping has cooling of the reaction fluid and temperature control ≤ 20 C, after dropping stirring 0.5 h; layered, for methylene chloride level (the upper layer) 12.0 kg (25% w/w) saturated sodium chloride solution agitation washing, the delaminated, taking methylene chloride level (lower) spare; to the 50 L reaction flask add 5.4 kg concentrated ammonia water, then slowly adding the dichloromethane layer solution, at room temperature (20 - 30 C) stirring the reaction 2 - 3 H of the; added to the reaction solution after 11.7 kg isopropanol, 35 C thermal insulation to continue stirring 1.5 h, then cooled to 15 - 20 C, stirring crystallization 6 h, then adding, for material 2.4 kg of cold isopropanol washing, 60 C drying under atmospheric pressure 5 - 10 the H, get-cutting to past cloth thick 3.1 kg; will-cutting to past cloth thick 3.1 kg and 7.36 kg methanol by adding 30 L glass reaction flask, stirring under heating to 60 - 70 C reflux, filtered to remove insoluble matter, then adding 3.1 kg purified water, the temperature slowly drops to 15 - 20 C, stirring crystallization 6 h, filtered, in order to 3.1 kg cold methanol/water (V/V=3:1, W/W=2.37: 1) in the solution, 60 C drying under atmospheric pressure 5 - 10 the H, get-cutting to [...] 2.2 kg. |
| 5.73 kg |
With ammonium hydroxide; In dichloromethane; at 0 - 30℃; for 1h; |
20kg of ammonia water was added to the reactor, and the dichloromethane solution of 6.0kg of Intermediate I (prepared by Example 2) was added dropwise at 0-15C for temperature control (6.0kg of Intermediate I was dissolved in 9.1kg of dichloromethane). After dropping, the temperature was raised to 25-30C for 1 hour. 28kg of purified water was added, and stirring was continued for 1 hour at 25-30C. Centrifuge, wash with purified water, and dry to obtain 6kg of crude product. Add 30kg of ethanol with a water content of 5%, be warming up to 70-80C to dissolve, cool to 20-25C for crystallization for 1 hour, centrifuge, rinse with 1.2kg of absolute ethanol, and dry to obtain 5.73kg of white solids, namely for valdecoxib. The purity is 99.6% (see Figure 2 for details). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping