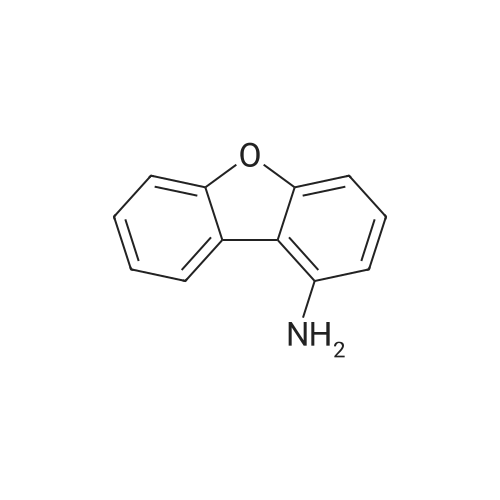
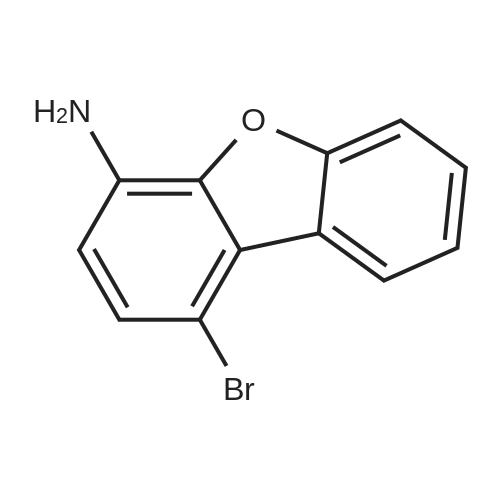
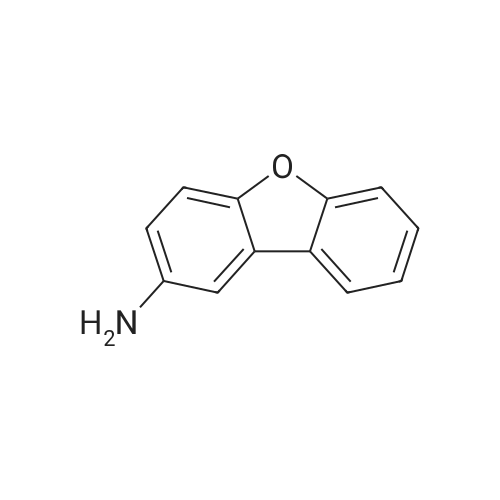
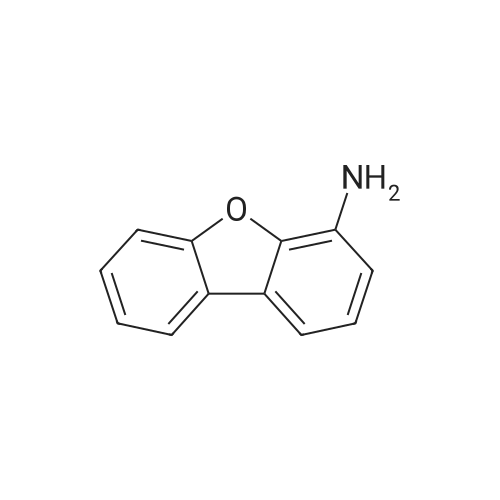
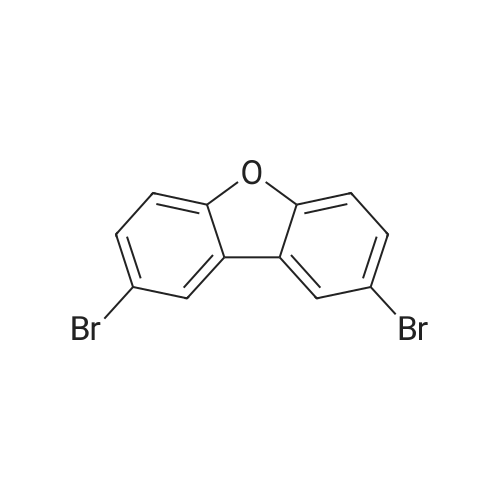
| 70% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 10h;Reflux; |
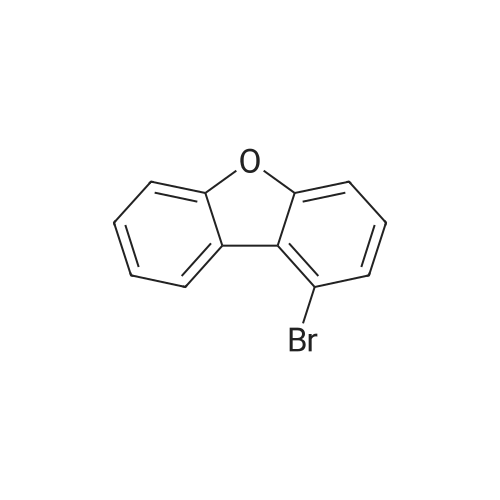
500ml round bottom flask reactor1-Bromo-dibenzofuran (20.0g, 0.081mmol), Bis (pinacolato) diboron (26.7g, 0.105mol),[1,1'-bis (diphenylphosphino) ferrocene] palladium in a Dijk (1.3g, 0.002mol),Potassium acetate (19.9g,0.202mol), into a 1,4-dioxane 200ml were stirred for 10 hours under reflux. After the completion of the reaction was filtered a pad of Celite. femaleAfter concentration under reduced pressure they were separated by liquid column and recrystallized with dichloromethane-heptane to give a (17.0g, 70%). |
| 70% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 10h;Reflux; |
<strong>[50548-45-3]1-bromodibenzofuran</strong> (20.0 g, 0.081 mmol) was charged in a 500 ml round bottom flask reactor,Bis (pinacol) diboron (26.7 g, 0.105 mol)[1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium (1.3 g, 0.002 mol)Potassium acetate (19.9 g, 0.202 mol), 200 ml of 1,4-dioxane and stirred under reflux for 10 hours.After the reaction was complete, the diatomaceous earth liner was filtered. The filtrate was concentrated under reduced pressure and then separated by column,And recrystallized from dichloromethane and heptane to obtain (17.0 g, 70%). |
| 70% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 1h;Reflux; |
In a 500-mL round-bottom flask reactor, <strong>[50548-45-3]1-bromodibenzofuran</strong> (20.0 g, 0.081 mmol), bis(pinacolato)diboron (26.7 g, 0.105 mol), [1,1?-bis(diphenylphosphino)ferrocene]dichloropalladium (1.3 g, 0.002 mol), potassium acetate (19.9 g, 0.202 mol), and 1,4-dioxane (200 ml) were stirred together for hrs under reflux. After completion of the reaction, filtration was performed through a celite pad. The filtrate was concentrated in a vacuum, purified by column chromatography, and recrystallized in dichloromethane and heptane to afford <Intermediate 4-a> (17.0 g, 70%). |
| 70% |
With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate; In 1,4-dioxane; for 10h;Reflux; |
In a 500-mL round-bottom flask reactor, <strong>[50548-45-3]1-bromodibenzofuran</strong> (20.0 g, 81 mmol), bis(pinacolato)diboron (26.7 g, 105 mmol), [1,1?-bis(diphenylphosphino)ferrocene]dichloropalladium (1.3 g, 0.002 mol), potassium acetate (19.9 g, 202 mmol), and 1,4-dioxane (200 ml) were stirred together for 10 hrs under reflux. Concentration in a vacuum was followed by column chromatographic purification. Recrystallization in dichloromethane and heptane afforded (17.0 g, 70%). |
| 70% |
With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate; In 1,4-dioxane; for 10h;Reflux; |
Intermediate 8-a was synthesized according to Scheme 60 below:<strong>[50548-45-3]1-bromodibenzofuran</strong> (20.0g, 0.081mmol), bis (pinacolato) diboron (26.7g, 0.105mol) in 500ml round bottom flask reactor,200 ml of [1,1'-bis (diphenylphosphino) ferrocene] dichloropalladium (1.3 g, 0.002 mol), potassium acetate (19.9 g, 0.202 mol) and 1,4-dioxane were added and stirred under reflux for 10 hours. . After the reaction was completed, the celite pad was filtered. The filtrate was concentrated under reduced pressure and column separatedRecrystallization from dichloromethane and heptane gave
|

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping