Alternatived Products of [ 505-54-4 ]
Product Details of [ 505-54-4 ]
| CAS No. : | 505-54-4 |
MDL No. : | MFCD00002746 |
| Formula : |
C16H30O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | QQHJDPROMQRDLA-UHFFFAOYSA-N |
| M.W : |
286.41
|
Pubchem ID : | 10459 |
| Synonyms : |
HDA;Thapsic Acid;α,ω-Hexadecanedioic Acid;1,16-Hexadecanedioic Acid;1,16-Hexadecadioic Acid;NSC 15164
|
Application In Synthesis of [ 505-54-4 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 505-54-4 ]
- 1
-
 [ 688763-60-2 ]
[ 688763-60-2 ]

-
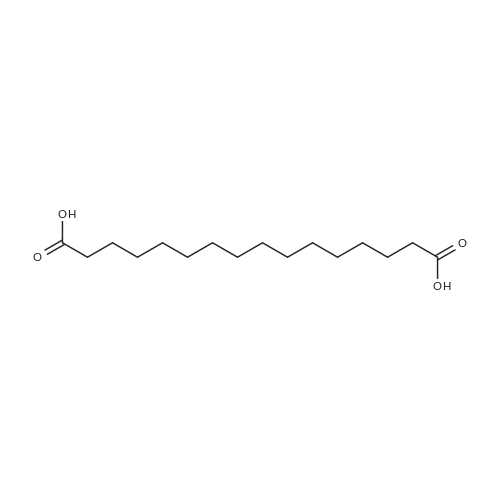 [ 505-54-4 ]
[ 505-54-4 ]

-
 [ 71989-23-6 ]
[ 71989-23-6 ]

-
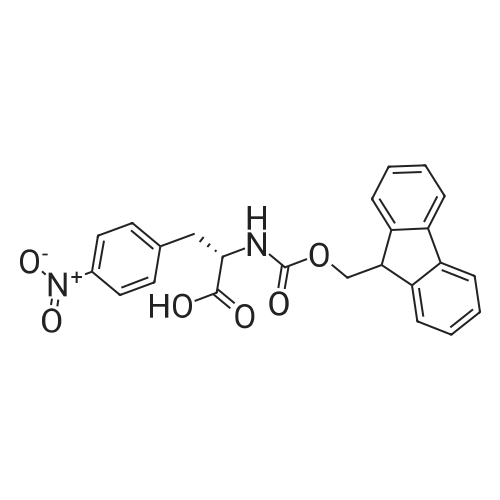 [ 95753-55-2 ]
[ 95753-55-2 ]

-
(S)-2-[(S)-3-Carbamoyl-2-(15-{(1S,2S)-1-[(S)-1-carbamoyl-2-(4-nitro-phenyl)-ethylcarbamoyl]-2-methyl-butylcarbamoyl}-pentadecanoylamino)-propionylamino]-3-phenyl-propionic acid
[ No CAS ]
- 2
-
 [ 25077-25-2 ]
[ 25077-25-2 ]

-
 [ 505-54-4 ]
[ 505-54-4 ]
Reference:
[1]Collection of Czechoslovak Chemical Communications,1953,vol. 18,p. 804,814
[2]Collection of Czechoslovak Chemical Communications,1959,vol. 24,p. 2484,2489
[3]Collection of Czechoslovak Chemical Communications,1959,vol. 24,p. 2484,2489
- 3
-
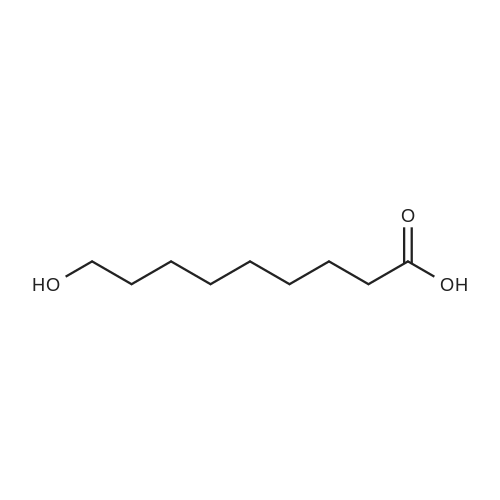 [ 3788-56-5 ]
[ 3788-56-5 ]

-
 [ 505-54-4 ]
[ 505-54-4 ]
- 4
-
 [ 505-54-4 ]
[ 505-54-4 ]

-
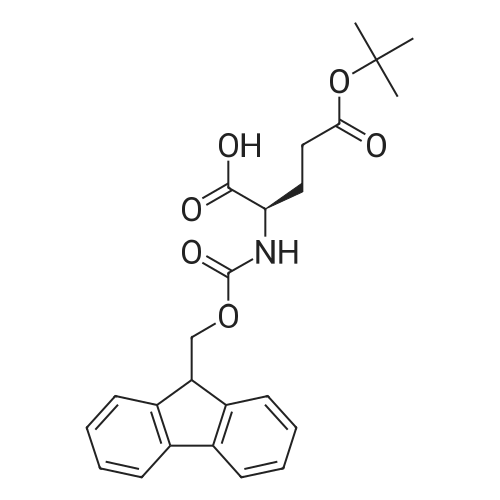 [ 104091-08-9 ]
[ 104091-08-9 ]

-
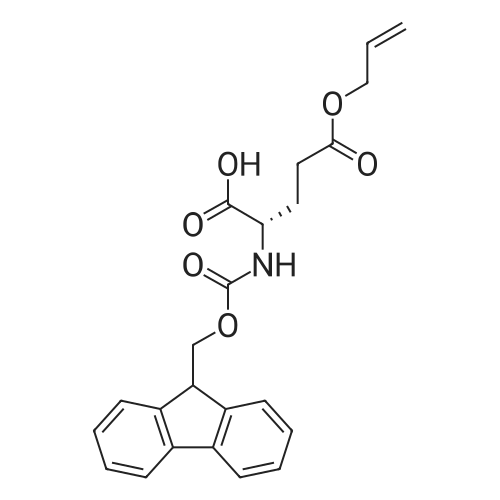 [ 133464-46-7 ]
[ 133464-46-7 ]

-
(S)-6-[(Diphenyl-p-tolyl-methyl)-amino]-2-(9H-fluoren-9-ylmethoxycarbonylamino)-hexanoic acid
[ No CAS ]

-
Nα-(9-fluorenylmethyloxycarbonyl)-Nγ-2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl-L-arginine
[ No CAS ]

-
C56H102N20O14
[ No CAS ]
| Yield | Reaction Conditions | Operation in experiment |
|
|
CycK(DE-Hdd)R4E (i.e. CycK(e-Hdd)R4E) was synthesized on Wang resin with a loading of 0.22 mmol/g. Fmoc-Glu(OAll)-OH (4 eq.) was loaded onto the resin (200 mg 44 muiotaetaomicron, 1 eq.) via Mitsunobo reaction employing triphenylphosphine (4 eq.) and diisopropylazodicarboxylate (4 eq.) in THF. The reaction was left to proceed for 2 h at RT on a shaker. Fmoc was removed by incubation in 20 % piperidine/NMP for 2 x 10 min. Arginine was coupled via Fmoc-Arg(Pbf)-OH (10 eq.) with HBTU (9.8 eq.) and DIPEA (20 eq.) in NMP for 30 min at RT and Fmoc was removed by incubation in 20 % piperidine/NMP unless noted otherwise. This procedure was repeated 3 times. Fmoc-Lys(Mtt)-OH (2 eq.) was coupled with COMU (1.8 eq.) and DIPEA (4 eq.) in NMP overnight. Fmoc was removed and OA11 was removed by incubation with Tetrakis(triphenylphosphine)palladium(0) (20 mg/100 muetaiotaomicron peptide) and dimethylaminoboran (100 mg/100 muetaiotaomicron peptide) in DCM for 20 min. Resin was washed with 10 % ethanolamine/DCM for 5 min twice followed by washes with DCM, MeOH, DCM and NMP. N-terminal to glutamic acid side chain cyclisation was carried out with 5 eq. PyAOP (521 g/mol) and 7.5 eq. of DIPEA (Triethylamin for cyclisation in solution) in NMP for 1 h. Side chain protection group Mtt was removed by incubation with 3.5 % TCA/DCM twice (yellow colour of resin and solution) followed by incubation with 3.5% TCA/2.5% TIS/DCM for 2h. Fmoc-D-Glu(OtBu) (2 eq.) was coupled with COMU (1.8 eq.) and DIPEA (4 eq.). Fmoc was removed and hexadecanedioic acid (1.2 eq.) was coupled with COMU (1 eq.). |
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping













