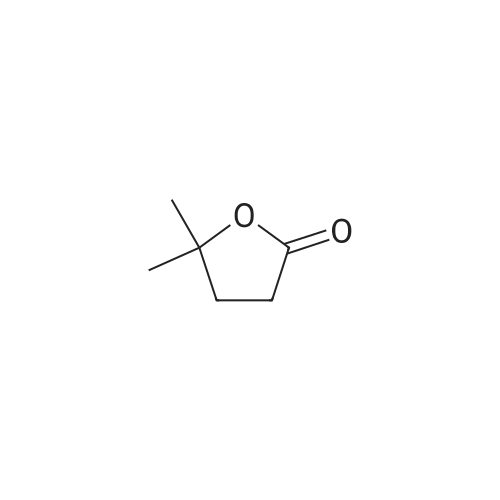
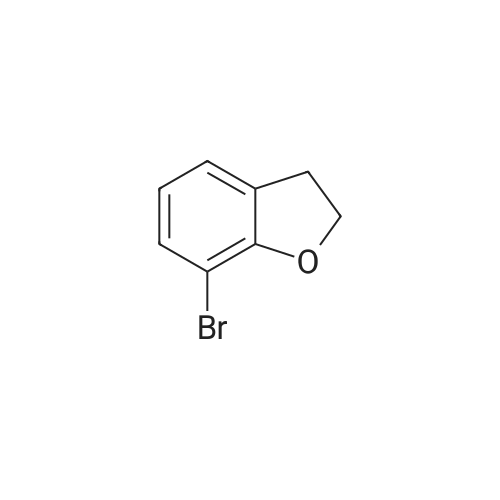
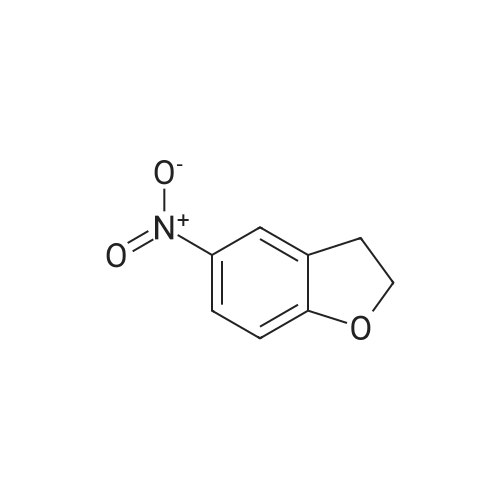
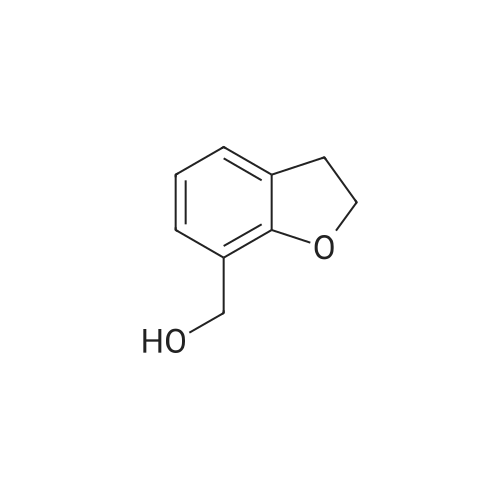
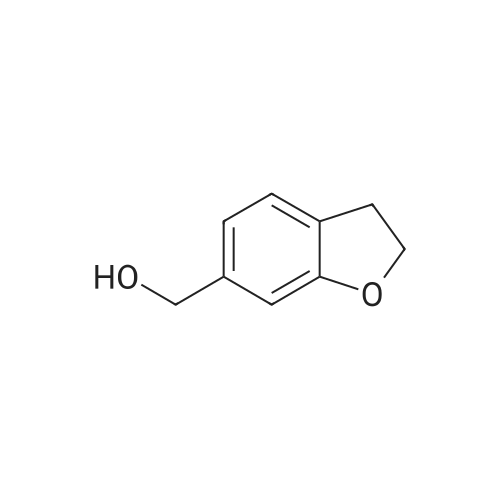
| 54.7% |
With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; In hexane; |
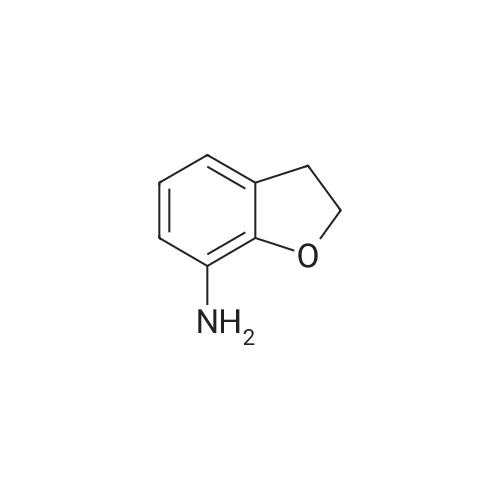
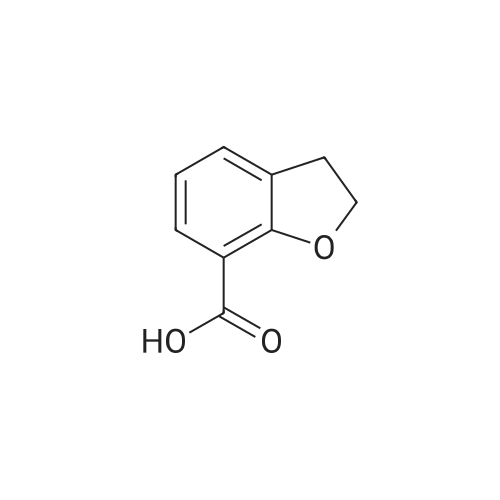
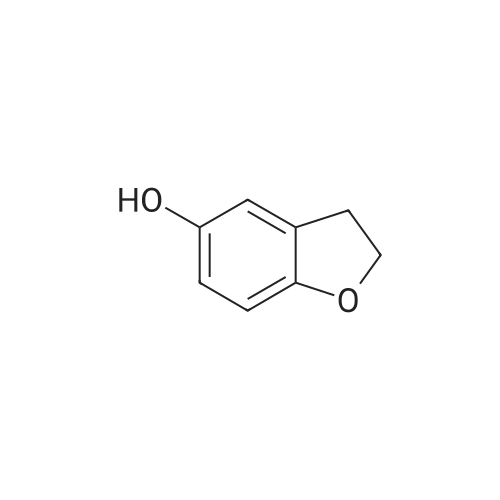
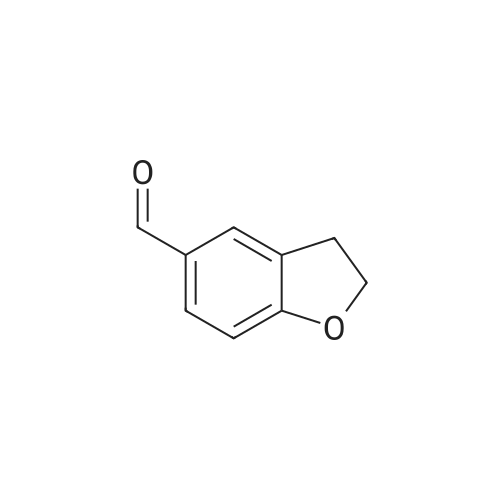
a. Preparation of 2,3-dihydrobenzo[b]furan-7-carboxylic acid (15a) To a solution of n-BuLi (1.7 M, 123 ml, 0.21 mol) in 400 ml of hexane at room temperature was added 24.3 g (0.21 mol) of N,N,N',N'-tetramethylethylenediamine (TMEDA), followed by a hexane (40 ml) solution of 2,3-dihydrobenzo[b]furan (14) (12.56 g, 0.11 mol). The mixture was stirred under argon at room temperature for 4 hours, and then poured into dry ice (pre-washing with anhydrous ether). After stirring at ambient temperature overnight, the mixture was diluted with water (300 ml), and the layers separated. The aqueous layer was acidified with conc. HCl to pH 1, cooled and the precipitate collected on a filter. This was recrystallized from CH2 Cl2 to give 15a (9.43 g, 54.7%) as a white solid, mp 167-169.5 C. |
| 54.7% |
With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; In hexane; |
a. Preparation of 2,3-dihydrobenzo[b]furan-7-carboxylic acid (15a) To a solution of n-BuLi (1.7 M, 123 ml, 0.21 mol) in 400 ml of hexane at room temperature was added 24.3 g (0.21 mol) of N,N,N',N'-tetramethylethylenediamine (TMEDA), followed by a hexane (40 ml) solution of 2,3-dihydrobenzo[b]furan (14) (12.56 g, 0.11 mol). The mixture was stirred under argon at room temperature for 4 hours, and then poured into dry ice (pre-washing with anhydrous ether). After stirring at ambient temperature overnight, the mixture was diluted with water (300 ml), and the layers separated. The aqueous layer was acidified with conc. HCl to pH 1, cooled and the precipitate collected on a filter. This was recrystallized from CH2Cl2 to give 15a (9.43 g, 54.7%) as a white solid, mp 167-169.5C. |
| 18% |
With n-butyllithium; N,N,N,N,-tetramethylethylenediamine; carbon dioxide; In tetrahydrofuran; sodium hydroxide; |
EXAMPLE 21 7-Carboxy-2,3-dihydrobenzofuran(22) To a stirred solution of 2,3-dihydrobenzofuran (1 g, 0.0083 mol) and dry TMEDA (1 eq.) in dry tetrahydrofuran (30 mL) at -10 C. under argon, was added n-BuLi (1.6 M hexane solution) (5 mL, 0.008 mol). The reaction mixture was maintained at this temperature for 20 min and then carbon dioxide was bubbled through the reaction mixture as it warmed to room temperature. The reaction mixture was concentrated in vacuo to afford a yellow oil. The oil was dissolved in 10% sodium hydroxide (100 mL), washed with methylene chloride (2*25 mL), and acidified with HCl. The white solid product was filtered, washed with water and air-dried to afford the carboxylic acid (22) (0.22 g, 18%). 1 H NMR (CDCl3) delta 7.35(1H,d), 7.11(1H,d), 6.59(1H,t), 4.37(2H,t), 2.99(2H,t). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping