| 86% |
|
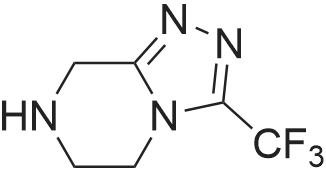
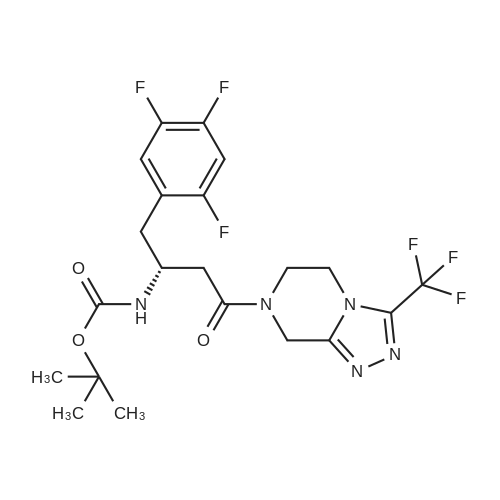
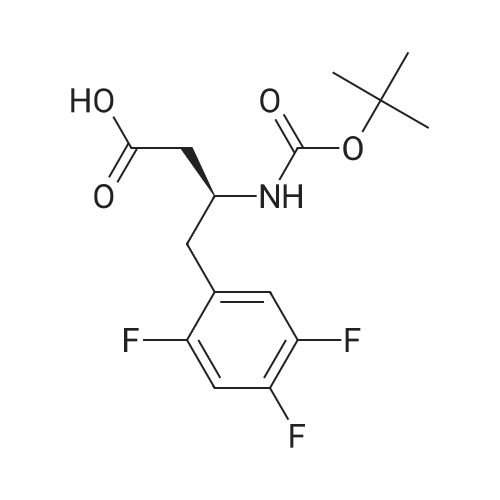
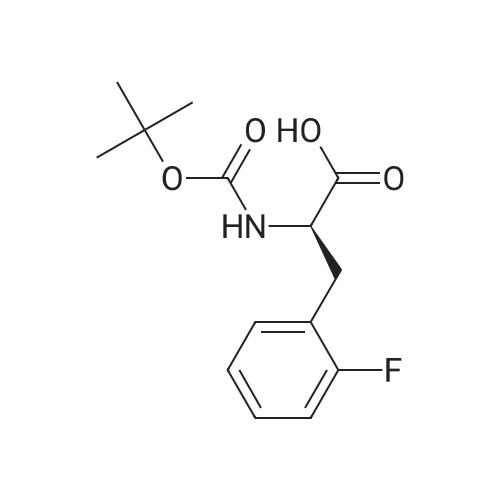
EXAMPLE 7 This example is about step 7 of the process of the invention, production of tert-butyl-4-oxo-4-(3-(trifluoromethyl)-5,6-dihydro-[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)-1-(2,4,5-trifluorophenyl)butan-2-yl-carbamate, compound (X) with R = tert-butyl (amide formation with EDC/N-HOBT) N-hydroxybenzotriazole (150 mg, 1.08mmol) is added at 0 C to a solution of (R)-3-(tert-butoxycarbonylamino)-4-(2,4,5-trifluorophenyl)butanoic acid (300 mg, 0.90 mmol) and <strong>[486460-21-3]3-(trifluoromethyl)-5,6,7,8-tetrahydro-[1,2,4]triazolo[4,3-a]pyrazine</strong> (170 mg, 0.90 mmol) in CH2Cl2 (10ml); after 10 minutes EDC (260 mg, 1.35mmol) is added. The mixture is stirred under nitrogen at room temperature for 4 hours monitoring by TLC (AcOEt). The reaction is quenched by addition of a saturated solution of NaHCO3 (5 mL); after phase separation the organic layer is washed with brine (5 mL), dried over sodium sulphate, concentrated to a residue and purified by flash chromatography eluting with AcOEt obtaining the title product (406 mg, 86%) as a off-white solid. 1H NMR (400 MHz, CDCl3) delta 7.14-7.04 (m, 1 H), 6.97-6.83 (m, 1 H), 5.32-5.24 (bs, 1 H), 5.16-5.00 (m, 1 H), 4.94 (s, 1 H), 4.33-3.94 (m, 5H), 3.02-2.62 (m, 4H) 1.38 (s, 9H). |
| 82% |
|
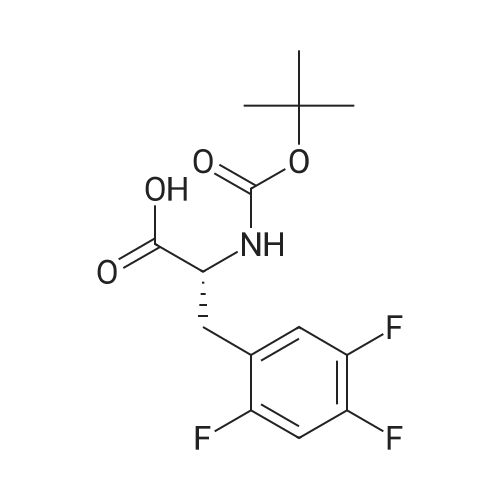
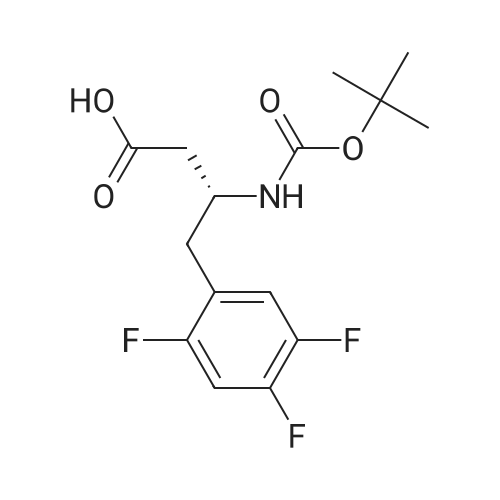
To a solution of (R)-3-[(tert-butyloxycarbonyl)amino]-4-(2,4,5-trifluorophenyl)- butanoic acid (1.36 g, 4.080 mmol) in acetonitrile (20 ml) was added Diisopropylethylamine (2.47 g, 19.12 mmol) and 1 ,1-Carbonyl diimidazole (0.94 g, 5.82 mmol) at room temperature. The reaction mixture was stirred for 30 min at room temperature. S-iTrifluoromethylJ-S.ej.e-tetrahydro-l ,2,3-triazolo[4,3- a]pyrazine (0.750 g, 3.88 mmol) was added to the above reaction mixture at room temperature. The reaction mixture was heated to 65-70C for 22 h. After completion of the reaction, the mixture was concentrated under vacuum and the crude mass was dissolved in ethyl acetate (15 ml) and washed with 5% aqueous NaHC03 solution followed by twice with water (2 x 30 ml). The ethyl acetate layer was concentrated under reduced pressure to get crude mass and recrystallized from mixture of 10 % ethyl acetate and petroleum ether (50 ml) to get 1.5 g (82%) of tert-butyl{(1 R)-3-oxo-1 -(2,4,5-trifluorobenzyl)-3-[3-(trifluoromethyl)-5-6- dihydro[1 ,2,4] triazole[4,3-a]pyrazin-7(8H)-yl]propyl}carbamate. |
| 81% |
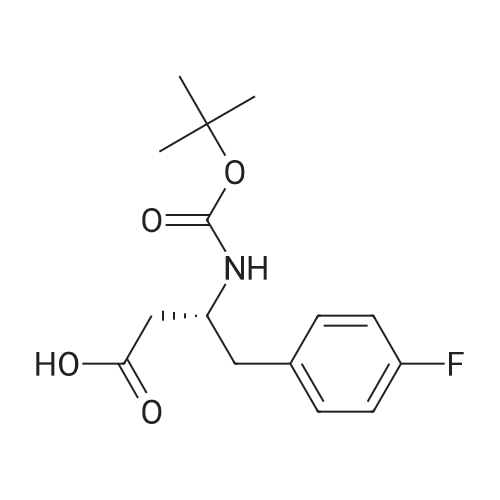
With hydrogenchloride; benzotriazol-1-ol; 1,2-dichloro-ethane; triethylamine; In N,N-dimethyl-formamide; at 20℃; for 15h; |
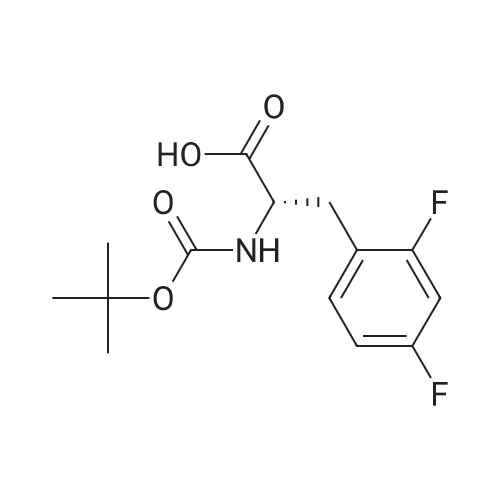
3.0 g of intermediate V (4.5 mmol) was dissolved in 25 mL of DMF,2.06 g of EDC.HCl (10.8 mmol) and1.46 g of HOBT (10.8 mmol) followed by 1.80 g of starting material X (9.0 mmol) and 1.30 mL of triethylamine (9.0 mmol)Stirred at room temperature for 15 h,Add water to extract the reaction,Ethyl acetate extraction, organic NaHCO3 aqueous solution and saturated brine,No waterNa2SO4. The solvent was evaporated under reduced pressure and the column chromatography (EtOAc / petroleum ether = 7: 3) gave the product as a white solid intermediate |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping