|
|
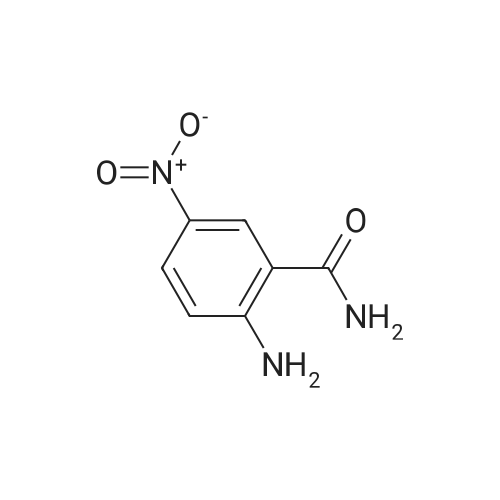
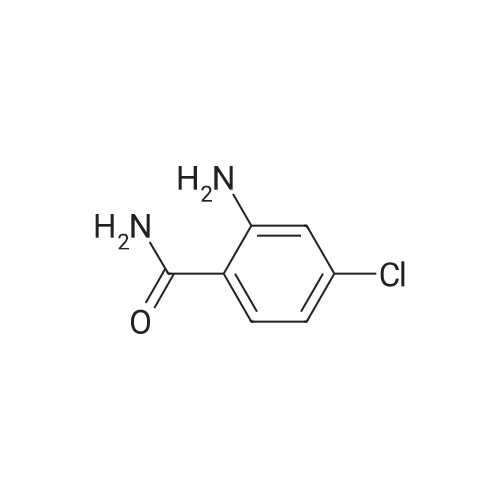
EXAMPLE 30 2'-Carbamyl-4'-nitrooxanilic acid ethyl ester. 59 <strong>[16313-65-8]<strong>[16313-65-8]2-Amino-5-nitrobenzamid</strong>e</strong> (5.43 g., 0.03 mole) is condensed with 3.7 ml. (0.033 mole) of ethyl oxalyl chloride in a manner similar to example 12, giving 6.54 g. of the title compound, m.p. 206-209 C., after crystallization from ethanol. Elemental Analysis for C11 H11 N3 O6: Calc'd: C, 46.98; H, 3.94; N, 14.94. Found: C, 46,68; H, 3.96; N, 15.12. |
|
|
EXAMPLE 47 2'-Carbamoyl-4'-nitrooxanilic acid ethyl ester. 59 <strong>[16313-65-8]<strong>[16313-65-8]2-Amino-5-nitrobenzamid</strong>e</strong> (5.43 g, 0.03 mole) is condensed with 3.7 ml (0.033 mole) of ethyl oxalyl chloride in a manner similar to example 3, giving 6.54 g of the title compound, m.p. 206-209 C., after crystallization from ethanol. Elemental Analysis for C11 H11 N3 O6: Calc'd: C, 46.98; H, 3.94; N, 14.94. Found: C, 46.68; H, 3.96; N, 15.12. |
|
With triethylamine; In tetrahydrofuran; at 0℃; for 1h; |
Step 2 To a solution of <strong>[16313-65-8]2-amino-5-nitrobenzamide</strong> (18.1 g) and triethylamine (15 mL) in THF (100 mL) was added dropwise a solution of ethyl chloroglyoxylate (14.4 g) in THF (20 mL) under ice-cooling, and the mixture was stirred at 0C for 1hr. 1N Hydrochloric acid was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer was washed with water, saturated aqueous sodium hydrogencarbonate and saturated brine, dried, and concentrated. The obtained crude crystals were washed with diisopropyl ether to give ethyl [2-(aminocarbonyl)-4-nitrophenyl]amino}(oxo)acetate (8.1 g). 1H-NMR (300MHz, DMSO-d6) delta: 1.37 (3H, t, J = 7.1 Hz), 3.33 (3H, s), 4.41 (2H, q, J = 7.1 Hz), 8.03 (1H, d, J = 8.8 Hz), 8.59 (1H, dd, J = 8.8, 2.7 Hz), 8.83 (1H, d, J = 2.2 Hz). |
| 8.1 g |
With triethylamine; In tetrahydrofuran; at 0℃; for 1h; |
Step 2 To a solution of <strong>[16313-65-8]2-amino-5-nitrobenzamide</strong> (18.10 g) and triethylamine (15 ml) in THF (100 mL) was added dropwise a solution of ethyl chloroglyoxylate (14.36 g) in THF (20 ml) under ice-cooling, and the mixture was stirred at 0 C. for 1 hr. 1N Hydrochloric acid was added to the reaction mixture and the mixture was extracted with ethyl acetate. The organic layer was washed with water, saturated aqueous sodium hydrogen carbonate and saturated brine, dried and concentrated. The obtained crude crystals were washed with diisopropy ether to give ethyl [2-(aminocarbonyl)-4-nitrophenyl]amino}(oxo)acetate (8.1 g). 1H NMR (300 MHz, DMSO-d6) delta: 1.4 (t, J=7.1 Hz, 2H) 3.3 (s, 3H) 4.4 (q, J=7.1 Hz, 2H) 8.0 (d, J=8.8 Hz, 1H) 8.6 (dd, J=8.8, 2.7 Hz, 1H) 8.8 (d, J=2.2 Hz, 1H) |
|
|
EXAMPLE 47 2'-Carbamoyl-4'-nitrooxanilic acid ethyl ester. 59 <strong>[16313-65-8]<strong>[16313-65-8]2-Amino-5-nitrobenzamid</strong>e</strong> (5.43 g, 0.03 mole) is condensed with 3.7 ml (0.033 mole) of ethyl oxalyl chloride in a manner similar to example 3, giving 6.54 g of the title compound, m.p. 206-209C., after crystallization from ethanol. Elemental Analysis for C11 H11 N3 O6: Calc'd: C, 46.98; H, 3.94; N, 14.94. Found: 46.68; H, 3.96; N, 15.12. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +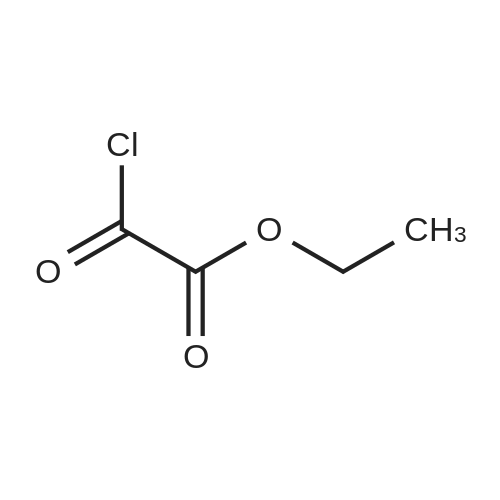

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping