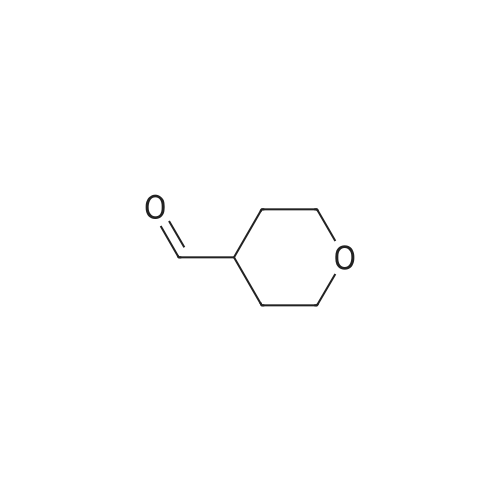
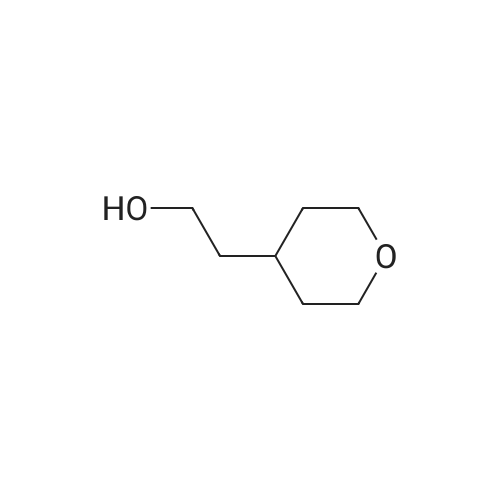
| 66.1% |
With lithium aluminium tetrahydride; In tetrahydrofuran; ethyl acetate; at 0℃; for 16h; |
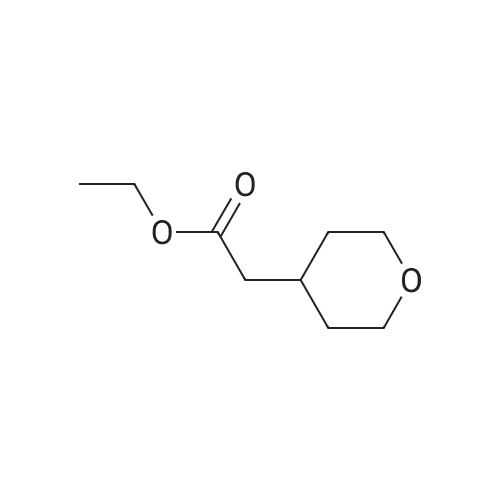
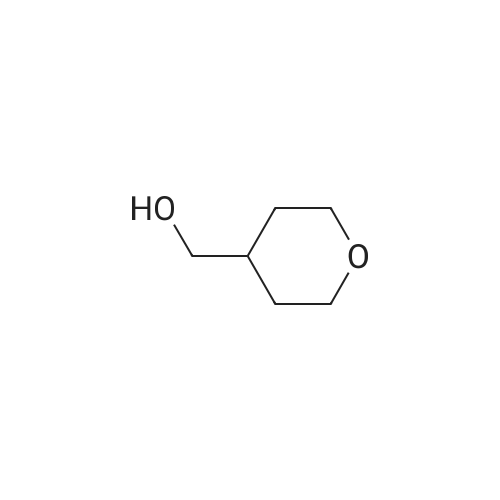
Lithium aluminum hydride (2M solution in THF, 40.66 ml, 81.3 mmol) was cooled at 0 C and a solution of <strong>[103260-44-2]ethyl 2-(tetrahydro-2H-pyran-4-yl)acetate</strong> (14.0 g, 81.3 mmol) in THF (70 ml) was added dropwise. Ethyl acetate (20 ml) was added to the reaction mixture dropwise at 0 C and the resulting mixture was allowed to stir for 16 h. The reaction mixture was filtered through Celite and the filtrate was concentrated to give crude compound. The crude material was purified by column chromatography using mobile phase 0-65% ethyl acetate in hexane to afford the title compound (66.1%). ?H NMR (400MHz, CDC13) & 5.71 (s, 1H), 4.18-4.15 (m, 2H), 3.81-3.75 (m, 4H), 3.05-3.02 (m, 2H), 2.37-2.34 (m, 2H), 1.32- 1.31 (m, 3H). |
|
|
To 15 mL of tetrahydrofuran (THF) at 0 0C was added LiAlH4 (0.28 g, 7.3 mmol). This mixture was stirred for 10 min then the ethyl tetrahydropyran-4-yl-acetate (Combi- Blocks Inc., 0.50 g, 2.9 mmol) was added. The reaction was stirred for 5 min at 0 0C then was allowed to warm to ambient temperature and was stirred for 90 min. The reaction was quenched with excess NaHSO4-IOH2O and was stirred for 60 min. The mixture was filtered through Celite. The filtrate was concentrated to give the title compound which was carried on without further purification. MS (DCI/NH3) m/z 131 (M+H)+. EPO <DP n="41"/> |
|
|
To a suspension of lithium aluminium hydride (11 g, 0.29 mol) in dry tetrahydrofuran (350 mL) at 0 C. was added a solution of (tetrahydro-pyran-4-yl)-acetic acid ethyl ester (25 g, 0.145 mol) in dry tetrahydrofuran (100 mL) dropwise. The resulting mixture was then refluxed for 16 h. After cooling to 0 C., the reaction mixture was quenched carefully by slow addition of a saturated sodium carbonate solution (50 mL). The mixture was decanted and the precipitate was washed with tetrahydrofuran (2×200 mL). The combined tetrahydrofuran layers were dried over anhydrous sodium sulfate and then concentrated in vacuo to afford 2-(tetrahydro-pyran-4-yl)-ethanol (13 g, 69%) as a yellow oil which was used in the next step without purification. |
|
With lithium aluminium tetrahydride; In tetrahydrofuran; at 0 - 13℃; for 18h; |
[00106] To a mixture of <strong>[103260-44-2]ethyl 2-(tetrahydro-2H-pyran-4-yl)acetate</strong> (20 g, 116 mmol) in anhydrous THF (300 mL) was added lithium aluminum hydride (8.8 g, 232 mmol) portionwise at 0 C. The mixture was stirred at 11-13 C for 18 h. TLC (petroleum ether: ethyl acetate = 3: 1) showed no starting material remaining. The mixture was quenched with water (9 mL), 10% aq. NaOH solution (9 mL) and water (18 mL) successively at 0 C, filtered and concentrated under reduced pressure to give crude 2-(tetrahydro-2H-pyran-4- yl)ethanol (11.7 g, 77%) as an oil, which was used for the next step directly without further purification. 1H NMR (CDC13, 400 MHz): delta 3.86-3.90 (m, 2H), 3.58-3.61 (t, J = 6.4 Hz, 2H), 3.32-3.35 (t, J = 11.6 Hz, 2H), 2.69-2.70 (m, 1H), 1.61-1.63 (m, 3H), 1.54-1.60 (m, 2H), 1.43-1.45 (m, 2H). |
|
|
Intermediate 32: 2-(Tetrahydro-2/-/-pyran-4-yl)ethanolTo an ice-cold solution of lithium aluminium hydride (12.6 ml, 2.3M solution in tetrahydrofuran) in dry tetrahydrofuran (20 ml) and under nitrogen, was added a solution of ethyl tetrahydro-2/-/-pyran-4-yl acetate (5g) in dry tetrahydrofuran dropwise over 10 minutes. Following the addition the reaction was heated to reflux, overnight. The reaction was cooled and diluted with diethyl ether (100 ml). A 5M aqueous solution of sodium hydroxide (-10 ml) was added cautiously to the reaction mixture until the effervescence ceased. The formed white precipitate was filtered off. The resulting filtrate was dried over potassium carbonate, filtered and concentrated in vacuo. This yielded the title compound as a colourless oil (3.3g). MS calcd for (C7H14O2)" = 130 MS found (electrospray): (M+H)+ = 1311 H NMR (DMSO): 4.35 (1 H, t), 3.80 (2H, m), 3.43 (2H, m), 3.25 (2H, m), 1.60 (1 H, m), 1.54 (2H, m), 1.35 (2H, m), 1.13 (2H, m). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping