| 76% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; |
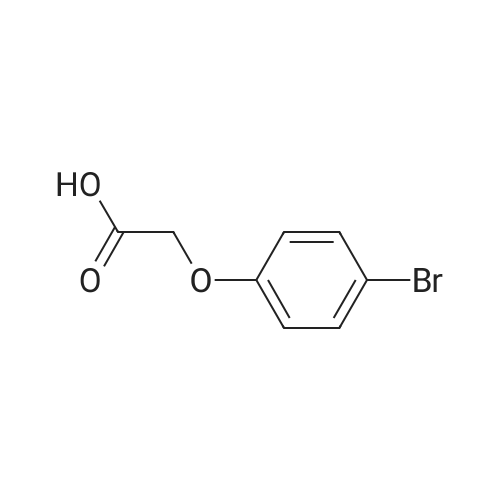
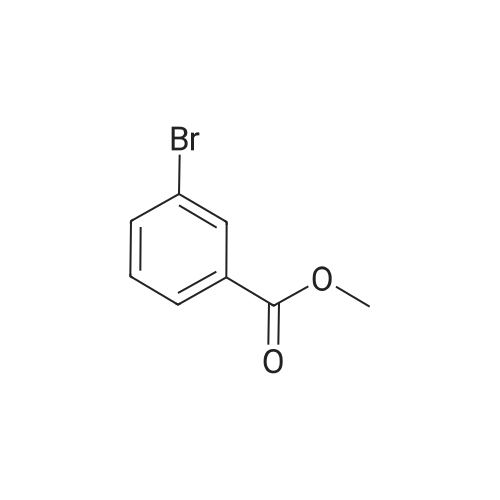
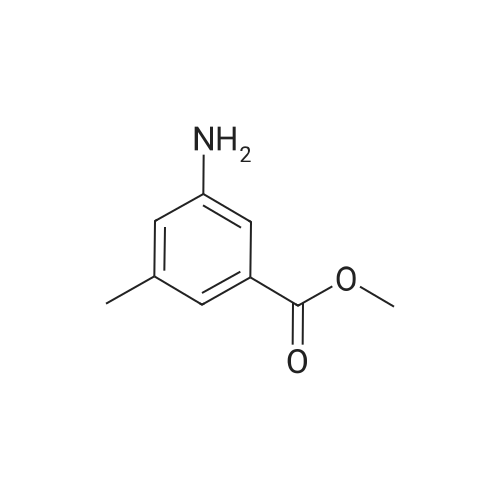
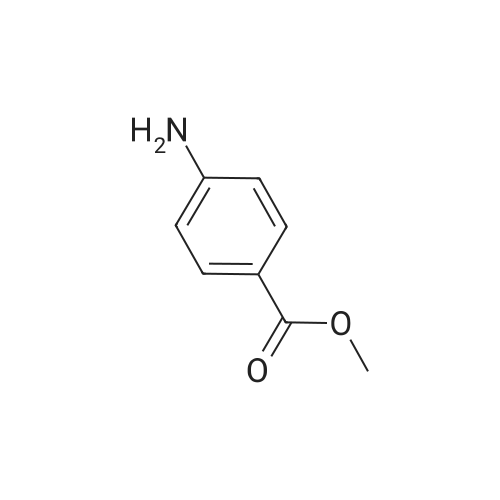
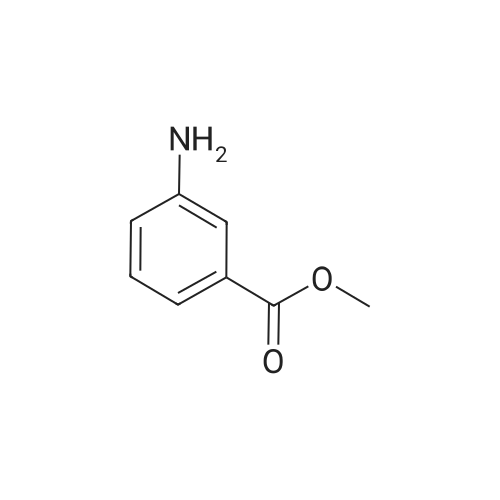
<Example 15> 3-[2-(4-bromo-phenoxy)-acetylamino]-benzoic acid methyl esterTo a solution of the <strong>[1878-91-7]4-bromophenoxy acetic acid</strong> (139 mg, 0.6 mmol) and amine (76 mg, 0.5 mmol) in DMF (5.0 mL) were added EDCHCl (144 mg, 0.75 mmol), HOBT (101 mg, 0.75 mmol), and DIPEA (0.13 mL, 0.75 mmol). The reaction mixture was stirred at room temperature overnight, and then partitioned between Ethyl acetat and brine. The organic phase was dried (anhydrous MgSO4), and concentrated. Purification by silica gel column chromatography (n-Hexane : Ethyl acetat : MeOH = 12 : 3: 1) gave 3-[2-(4-bromo-phenoxy)- acerylamino]-benzoic acid methyl ester as a white solid (138 mg, 76% yield). :. . 1H-NMR (CDCl3,.300Hz). . 8.32 (IH5 S5MI)5 8.06 (IH5 m, aromatic)5-7.99 (IH5 m5 aromatic), 7.84.(IH5 m, aromatic), 7.42-7.47 (3H5 m, aromatic), 6.89 (2H, m, aromatic), 4.59 (2H, s, OCH2CO), 3.92 (3H, s, OCH3). |
| 76% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; |
To a solution of the <strong>[1878-91-7]4-bromophenoxy acetic acid</strong> (139 mg, 0.6 mmol) and amine (76 mg, 0.5 mmol) in DMF (5.0 mL) were added EDCHCl (144 mg, 0.75 mmol), HOBT (101 mg, 0.75 mmol), and DIPEA (0.13 mL, 0.75 mmol). The reaction mixture was stirred at room temperature overnight and then partitioned between Ethyl acetate and brine. The organic phase was dried (anhydrous MgSO4), and concentrated. Purification by silica gel column chromatography (n-Hexane:Ethyl acetate:MeOH=12:3:1) gave 3-[2-(4-bromo-phenoxy)-acetylamino]-benzoic acid methyl ester as a white solid (138 mg, 76% yield). 1H-NMR (CDCl3, 300 Hz) 8.32 (1H, s, NH), 8.06 (1H, m, aromatic), 7.99 (1H, m, aromatic), 7.84 (1H, m, aromatic), 7.42-7.47 (3H, m, aromatic), 6.89 (2H, m, aromatic), 4.59 (2H, s, OCH2CO), 3.92 (3H, s, OCH3). |
| 56% |
With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine; In N,N-dimethyl-formamide; at 20℃; for 48h;Inert atmosphere; |
EDCI (1.5 eq.) and HOBt (1.5 eq.) were added to a solution of (4-bromo-phenoxy)-acetic acid 2 (1.0 eq.), 3-amino-benzoic acid methyl ester (1.5 eq.), and DIPEA (1.5 eq.) in DMF, and the reaction mixture was stirred for 48 h at RT. After evaporation of the DMF under reduced pressure, the reaction mixture was diluted with AcOEt and sequentially washed with 0.1 M HCl solution, aqueous sodium bicarbonate, and brine and dried over anhydrous MgSO4. The solvent was filtered and evaporated under reduced pressure to produce a crude solid, which was washed several times with diethyl ether to give the pure desired compound 3 (56% yield). Rf = 0.73 (n-hexane:AcOEt:MeOH = 6:3:1); NMR 1H (CDCl3, 300 MHz), delta (ppm): 3.92 (s, 3H, -OCH3), 4.59 (s, 2H, -OCH2CO-), 6.89 (m, 2H, ArH), 7.42-7.47 (m, 3H, ArH), 7.84 (m, 1H, ArH), 7.99 (m, 1H, ArH), 8.06 (1H, m, ArH), and 8.32 (1H, s, -NH-). Data consistent with the literature 3. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping