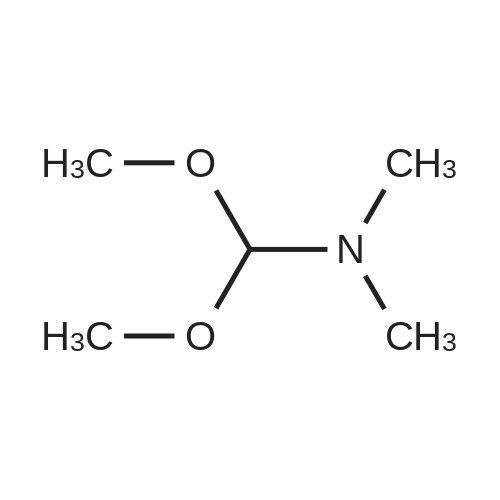
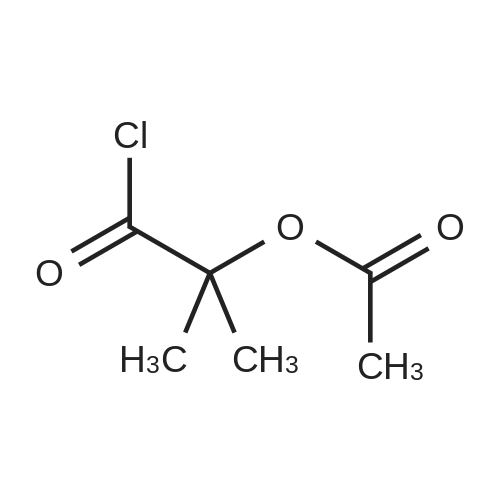
Alternatived Products of [ 4506-66-5 ]
Product Details of [ 4506-66-5 ]
CAS No. : 4506-66-5
MDL No. : MFCD00012970
Formula :
C6 H14 Cl4 N4
Boiling Point : -
Linear Structure Formula : -
InChI Key : BZDGCIJWPWHAOF-UHFFFAOYSA-N
M.W :
284.01
Pubchem ID : 78260
Synonyms :
Chemical Name : Benzene-1,2,4,5-tetraamine tetrahydrochloride
Calculated chemistry of [ 4506-66-5 ] Expand+
Physicochemical Properties
Num. heavy atoms : 14
Num. arom. heavy atoms : 6
Fraction Csp3 : 0.0
Num. rotatable bonds : 0
Num. H-bond acceptors : 0.0
Num. H-bond donors : 4.0
Molar Refractivity : 71.92
TPSA : 104.08 ?2
Pharmacokinetics
GI absorption : High
BBB permeant : No
P-gp substrate : No
CYP1A2 inhibitor : No
CYP2C19 inhibitor : No
CYP2C9 inhibitor : Yes
CYP2D6 inhibitor : No
CYP3A4 inhibitor : No
Log Kp (skin permeation) : -6.33 cm/s
Lipophilicity
Log Po/w (iLOGP) : 0.0
Log Po/w (XLOGP3) : 2.4
Log Po/w (WLOGP) : 3.26
Log Po/w (MLOGP) : 0.9
Log Po/w (SILICOS-IT) : -1.01
Consensus Log Po/w : 1.11
Druglikeness
Lipinski : 0.0
Ghose : None
Veber : 0.0
Egan : 0.0
Muegge : 0.0
Bioavailability Score : 0.55
Water Solubility
Log S (ESOL) : -3.43
Solubility : 0.106 mg/ml ; 0.000372 mol/l
Class : Soluble
Log S (Ali) : -4.23
Solubility : 0.0168 mg/ml ; 0.0000592 mol/l
Class : Moderately soluble
Log S (SILICOS-IT) : -0.93
Solubility : 33.1 mg/ml ; 0.117 mol/l
Class : Soluble
Medicinal Chemistry
PAINS : 0.0 alert
Brenk : 1.0 alert
Leadlikeness : 0.0
Synthetic accessibility : 1.46
Application In Synthesis of [ 4506-66-5 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
Downstream synthetic route of [ 4506-66-5 ]
1
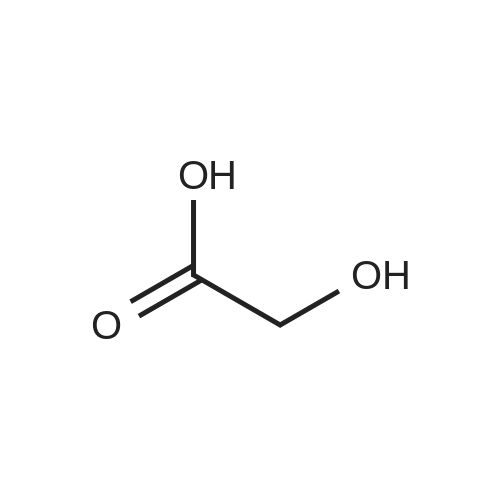
[ 64-18-6 ]
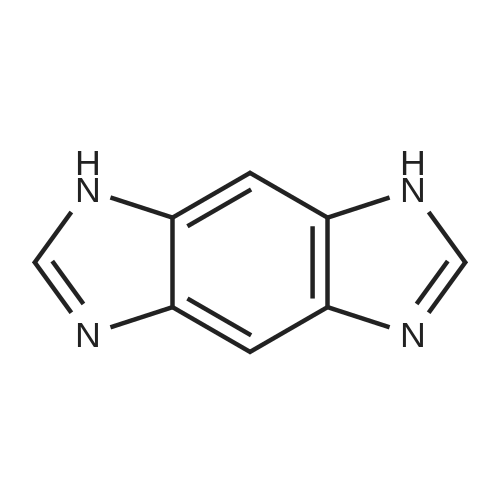
[ 4506-66-5 ]
[ 50738-59-5 ]
Yield Reaction Conditions Operation in experiment
at 20 - 100℃; for 36h;
To a 50 mL round bottom flask was added 1,2,4,5-tetraaminobenzene hydrochloride 1 (284 mg), formic acid (10 mL) at room temperature. The reaction was heated to 100 C for 36 hours. Cooled to room temperature, neutralized to neutral with NaOH solution, filtered and dried. The precipitate was transferred to 50 mL standard Schlenk vials, NaH (80 mg, 60%), 5 mL of toluene, followed by heating to 110 C under nitrogen. After cooling to room temperature, 1-bromobutane (1 mL) and DMF (5 mL) were added, and the mixture was heated to 110C for 4 hours to disappear the starting material. After cooling to room temperature, the inorganic salt was removed by filtration and recrystallized from methylene chloride-methanol to give tetrabutylbenzobisimidazole bromide salt 3a. Yield: 530 mg, 98%
Reference:
[1]Organic and Biomolecular Chemistry,2010,vol. 8,p. 3149 - 3156
[2]Journal of the American Chemical Society,2005,vol. 127,p. 12496 - 12497
[3]Journal of the American Chemical Society,2012,vol. 134,p. 17846 - 17849
[4]Chemical Communications,2019,vol. 55,p. 1786 - 1789
[5]Journal of the Chemical Society,1930,p. 1409,1413
[6]Chemical Communications,2009,p. 3750 - 3752
[7]Chemical Communications,2011,vol. 47,p. 7686 - 7688
[8]ChemCatChem,2015,vol. 7,p. 2248 - 2254
[9]Patent: CN106046057,2016,A .Location in patent: Paragraph 0064; 0065; 0066; 0067; 0068; 0069
2
[ 79-14-1 ]
[ 4506-66-5 ]
[ 112655-94-4 ]
3
[ 4506-66-5 ]
[ 517-21-5 ]
[ 99584-28-8 ]
4
[ 127-09-3 ]
[ 4506-66-5 ]
[ 108-24-7 ]
[ 96677-95-1 ]
5
[ 4506-66-5 ]
[ 108-24-7 ]
[ 17377-07-0 ]
6
[ 127-09-3 ]
[ 4506-66-5 ]
[ 431-03-8 ]
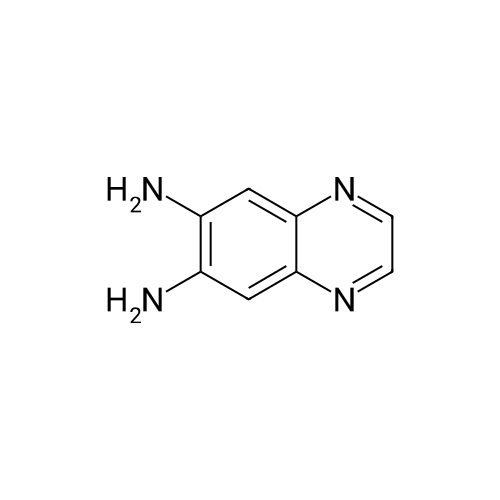
6,7-diamino-2,3-dimethylquinoxaline
[ No CAS ]
7
[ 4506-66-5 ]
[ 431-03-8 ]
[ 111076-18-7 ]
8
[ 4506-66-5 ]
[ 79-11-8 ]
[ 117044-72-1 ]
9
[ 50-00-0 ]
[ 4506-66-5 ]
[ 104779-70-6 ]
10
[ 1074-12-0 ]
[ 4506-66-5 ]
[ 111076-17-6 ]
11
[ 4506-66-5 ]
17,18-dioxo-5,10,15,20-tetraphenylchlorin
[ No CAS ]
C94 H58 N12
[ No CAS ]
12
[ 4506-66-5 ]
5,10,15,20-tetrakis(3,5-di-tert-butylphenyl)porphyrin-2,3-dione
[ No CAS ]
H2 (P)-TA-(P)H2
[ No CAS ]
13
[ 4506-66-5 ]
[ 103-71-9 ]
1,2,4,5-tetrakis(N'-phenylureido)benzene
[ No CAS ]
14
[ 27318-90-7 ]
[ 4506-66-5 ]
5,10,15,20-tetrakis(3,5-di-tert-butylphenyl)porphyrin-2,3-dione
[ No CAS ]
C94 H100 N10
[ No CAS ]
15
[ 27318-90-7 ]
[ 4506-66-5 ]
C82 H94 N8 O4
[ No CAS ]
C100 H102 N14 O2
[ No CAS ]
16
[ 4506-66-5 ]
[ 4637-24-5 ]
1,2,4,5-Tetrakis<(dimethylaminomethylene)amino>benzene dihydrochloride
[ No CAS ]
17
[ 4506-66-5 ]
[ 68-12-2 ]
Benzo<1,2-d;4,5-d'>diimidazole hydrochloride
[ No CAS ]
18
[ 4506-66-5 ]
[ 199275-03-1 ]
2,3,7,8-Tetrakis-[(triisopropylsilanyl)-ethynyl]-pyrazino[2,3-g]quinoxaline
[ No CAS ]
19
[ 4506-66-5 ]
132 -oxopyropheophorbide
[ No CAS ]
C74 H70 N12 O4
[ No CAS ]
C74 H70 N12 O5
[ No CAS ]
20
[ 4506-66-5 ]
[ 40635-66-3 ]
1,2,4,5-tetrakis(2-acetoxy-2-methylpropanamido)benzene
[ No CAS ]
Categories

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping