| 90.4% |
|
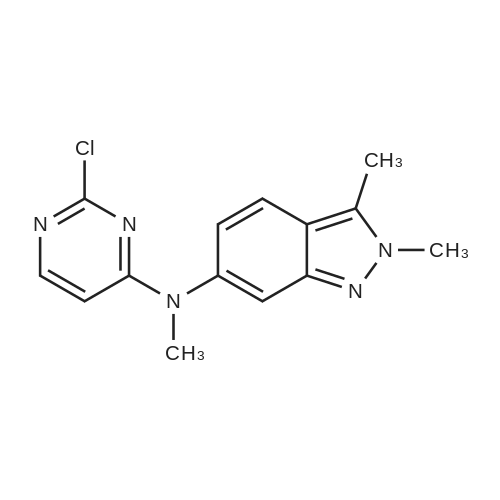
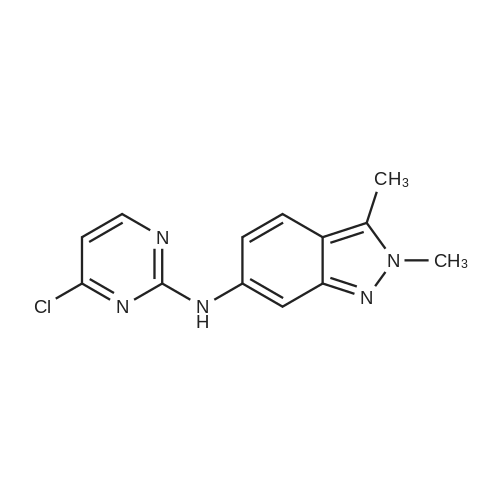
A 3L 3-necked flask equipped with air-driven mechanical stirrer, thermometer, addition funnel and nitrogen inlet/outlet was charged with DMF (272 ml_, 5 volumes) and the product of Intermediate Example 3 (54.4 g, 0.20 mol, 1.0 equiv) with stirring. The reaction mixture was further charged with cesium carbonate (194.5 g, 0.60 mol, 3.0 equiv) while maintaining the reaction temperature between 20 ~ 25C. The reaction mixture was stirred at 20 ~ 25C for 10 minutes, lodomethane (45.1 g, 0.32 mol, 1.6 equiv) was charged over ~ 10 minutes while maintaining the temperature 20 ~ 3O0C. The reaction mixture was stirred at 20 ~ 300C (Typically, the reaction is complete in 1 ~2 hours). Deionized H2O (925 ml_, 17 volumes) was added over ~ 30 minutes while maintaining the temperature at 25 ~ 400C. The reaction mixture was stirred at 20 ~ 25C for 40 minutes. The product was isolated by filtration and then the filter cake washed with H2O / DMF (6 : 1 , 252 ml_, 4.6 volumes). The wet cake was dried under vacuum at 40 ~ 45C and lambda/-(2-chloropyhmidin-4-yl)-lambda/,2,3-trimethyl-2/-/-indazol-6-amine (51.7 g, 90.4%) was isolated as a yellow solid. 1H NMR (400 MHz, DMSO-d6) delta 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, J = 7.0 Hz, 1 H), 7.50 (d, J = 1.0 Hz, 1 H), 6.88 (m, 1 H), 6.24 (d, J = 6.2 Hz, 1 H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 89.1% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; for 5h; |
To a stirred solution of the intermediate 4a (6.50 g, 24 mmol) in DMF (30 mL) was added Cs2CO3 (10.00 g, 31 mmol) and CH3I (5.70 g, 40 mmol) at room temperature. The mixture was stirred at rt for 5 h. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via fitration and washed with water. The precipitate was dried to afford 5a as an off-white solid (5.90 g, 89.1% yield). Mp: 172~173 C. 1HNMR (400MHz, DMSO-d6) delta: 2.62 (s, 3H), 3.41 (s, 3H), 4.05 (s, 3H), 6.23 (d, J=6.0 Hz, 1H), 6.87 (d, J=8.8 Hz, 1H), 7.49 (s, 1H), 7.80 (d, J=8.8 Hz, 1H), 7.93 (d, J=6.4 Hz, 1H). |
| 85.7% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃; for 2h; |
The suspension of ISO-IM1 (1g, 3.6 mmol), CH3I (0.76g, 5.4 mmol) and Cs2CO3 (2.34g, 7.2 mmol) in DMF (15 mL) was stirred for 2 h at room temperature.Then to the reaction mixture was added 100 mL H2O, filtered and dried to afford ISO-IM2 (0.9 g, 85.7%) as a yellow solid. 1H NMR (300 MHz, DMSO-d6) (delta, ppm):8.28 (d, J = 5.1 Hz, 1H), 7.62 (d, J = 8.8 Hz, 1H), 7.39 (s, 1H), 6.87 (d, J = 8.8 Hz,1H), 6.80 (d, J = 5.1Hz, 1H), 4.03 (s, 3H), 3.47 (s, 3H), 2.59 (s, 3H). |
| 83% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃;Product distribution / selectivity; |
To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 ml) was added Cs2CO3 (7.44 g, 2 eqv.) and iodomethane (1.84 ml, 1.1 eqv.) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford /V-(2-chloropyrimidin-4-yl)- Lambda/,2,3-trimethyl-2H-indazol-6-amine as an off-white solid (6.43 g, 83%). 1H NMR (400 MHz, DMSO-de) delta 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, J = 7.0 Hz, 1 H), 7.50 (d, J = 1.0 <n="31"/>Hz, 1 H), 6.88 (m, 1 H), 6.24 (d, J= 6.2 Hz, 1 H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 83% |
With caesium carbonate; In DMF (N,N-dimethyl-formamide); at 20 - 30℃; for 1 - 2h; |
To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 mi) was added Cs2CO3 (7.44 g, 2 eqv. ) and iodomethane (1.84 [ML,] 1.1 eqv. ) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford [N- (2-CHLOROPYRIMIDIN-4-] [YL)-N,] 2, 3-trimethyl-2H-indazol-6-amine as an off-white solid (6.43 [G,] 83%).'H NMR (400 MHz, [DMSO-D6)] [8] 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, [J =] 7.0 Hz, 1 H), 7.50 [(D, J =] 1.0 Hz, 1 H), 6.88 (m, 1 H), 6.24 (d, J = 6.2 Hz, 1 H), 4.06 (s, [3H),] 3.42 (s, [3H),] 2.62 (s, [3H).] MS (ES+, m/z) 288 (M+H) |
| 83 - 90.4% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 30℃;Product distribution / selectivity; |
To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 ml) was added Cs2CO3 (7.44 g, 2 eqv.) and iodomethane (1.84 ml, 1.1 eqv.) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford Lambda/-(2-chloropyrimidin-4-yl)- /V,2,3-trimethyl-2H-indazol-6-amine as an off-white solid (6.43 g, 83%). 1H NMR (400 MHz, DMSO-d6) delta 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, J = 7.0 Hz, 1 H), 7.50 (d, J = 1.0 Hz, 1 H), 6.88 (m, 1 H), 6.24 (d, J = 6.2 Hz, 1 H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H).; A 3L 3-necked flask equipped with air-driven mechanical stirrer, thermometer, addition funnel and nitrogen inlet/outlet was charged with DMF (272 ml_, 5 volumes) and the product of Intermediate Example 3 (54.4 g, 0.20 mol, 1.0 equiv) with stirring. The reaction mixture was further charged with cesium carbonate (194.5 g, 0.60 mol, 3.0 equiv) while maintaining the reaction temperature between 20 ~ 25 0C. The reaction mixture was stirred at 20 ~ 25 0C for 10 minutes, lodomethane (45.1 g, 0.32 mol, 1.6 equiv) was charged over ~ 10 minutes while maintaining the temperature 20 ~ 3O0C. The reaction mixture was stirred at 20 ~ 30 0C (Typically, the reaction is complete in 1 ~ 2 hours). Deionized H2O (925 ml_, 17 volumes) was added over ~ 30 minutes while maintaining the temperature at 25 ~ 40 0C. The reaction mixture was stirred at 20 ~ 25 0C for 40 minutes. The product was isolated by filtration and then the filter cake washed with H2O / DMF (6 : 1 , 252 ml_, 4.6 volumes). The wet cake was dried under vacuum at 40 ~ 45 0C and Lambda/-(2-chloropyrimidin-4-yl)-Lambda/,2,3-trimethyl-2H- indazol-6-amine (51.7 g, 90.4%) was isolated as a yellow solid. 1H NMR (400 MHz, DMSO-d6) delta 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, J = 7.0 Hz, 1 H), 7.50 (d, J = 1.0 Hz, 1 H), 6.88 (m, 1 H), 6.24 (d, J = 6.2 Hz, 1 H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 83% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃;Inert atmosphere; |
Intermediate Example 13 Preparation of N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine To a stirred solution of the Intermediate 12 (7.37 g) in DMF (50 ml) was added C2CO3 (7.44 g, 2 eqv.) and MeI (1.84 ml, 1.1 eqv.) at room temperature. Mixture was stirred at rt for overnight. The reaction mixture was poured into ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine as an off-white solid (6.43 g, 83%). 1H NMR (400 MHz, d6DMSO) delta 7.94 (d, J = 6.0 Hz, 1H), 7.80 (d, J = 7.0 Hz, 1H), 7.50 (d, J = 1.0 Hz, 1H), 6.88 (m, 1H), 6.24 (d, J = 6.2 Hz, 1H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 83% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃;Product distribution / selectivity; |
To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 ml) was added Cs2CO3 (7.44 g, 2 eqv.) and iodomethane (1.84 ml, 1.1 eqv.) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6-amine as an off-white solid (6.43 g, 83%). 1H NMR (400 MHz, DMSO-d6) delta 7.94 (d, J=6.0 Hz, 1H), 7.80 (d, J=7.0 Hz, 1H), 7.50 (d, J=1.0 Hz, 1H), 6.88 (m, 1H), 6.24 (d, J=6.2 Hz, 1H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 83 - 90.4% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20 - 30℃;Product distribution / selectivity; |
Intermediate Example 4 Preparation of N-(2-chloropyrimidin-4-yi )-N,2,3-trimethyl-2H-indazol-6-amine Procedure 1 To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 ml) was added Cs2C03 (7.44 g, 2 eqv. ) and iodomethane (1.84 ml, 1.1 eqv. ) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford N-(2-chloropyrimidin-4-yl)-N,2,3-trimethyl-2H-indazol-6- amine as an off-white solid (6.43 g, 83%).; Procedure 2 A 3L 3-necked flask equipped with air-driven mechanical stirrer, thermometer, addition funnel and nitrogen inlet/outlet was charged with DMF (272 mL, 5 volumes) and the product of Intermediate Example 3 (54.4 g, 0.20 mol, 1.0 equiv) with stirring. The reaction mixture was further charged with cesium carbonate (194.5 g, 0.60 mol, 3.0 equiv) while maintaining the reaction temperature between 20 - 25 C. The reaction mixture was stirred at 20 - 25 C for 10 minutes. lodomethane (45.1 g, 0.32 mol, 1.6 equiv) was charged over - 10 minutes while maintaining the temperature 20 - 30C. The reaction mixture was stirred at 20 - 30 C (Typically, the reaction is complete in 1 - 2 hours). Deionized H20 (925 mL, 17 volumes) was added over - 30 minutes while maintaining the temperature at 25 - 40 C. The reaction mixture was stirred at 20 - 25 C for 40 minutes. The product was isolated by filtration and then the filter cake washed with H2O / DMF (6 : 1, 252 mL, 4.6 volumes). The wet cake was dried under vacuum at 40 - 45 C and N-(2- chloropyrimidin-4-yl) -N,2,3-trimethyl-2H-indazol-6-amine (51.7 g, 90.4%) was isolated as a yellow solid. |
| 83% |
With caesium carbonate; In N,N-dimethyl-formamide; at 20℃;Product distribution / selectivity; |
To a stirred solution of the product of Intermediate Example 3 (7.37 g) in DMF (50 ml) was added Cs2CO3 (7.44 g, 2 eqv.) and iodomethane (1.84 ml, 1.1 eqv.) at room temperature. The mixture was stirred at rt overnight. The reaction mixture was then poured into an ice-water bath, and the precipitate was collected via filtration and washed with water. The precipitate was air-dried to afford lambda/-(2-chloropyrimidin-4-yl)- lambda/,2,3-thmethyl-2H-indazol-6-amine as an off-white solid (6.43 g, 83%). 1H NMR (400 MHz, DMSO-d6) delta 7.94 (d, J = 6.0 Hz, 1 H), 7.80 (d, J = 7.0 Hz, 1 H), 7.50 (d, J = 1.0 Hz, <n="34"/>1 H), 6.88 (m, 1 H), 6.24 (d, J = 6.2 Hz, 1 H), 4.06 (s, 3H), 3.42 (s, 3H), 2.62 (s, 3H). MS (ES+, m/z) 288 (M+H). |
| 80% |
|
The previous product, 2 1.00 g, and cesium carbonate, 2.40 g, were added to 50 mL of N,N-dimethylformamide.After reacting for 20 minutes at room temperature, then slowly adding 0.78 g of methyl iodide,After the addition, the reaction was carried out at room temperature for 2 hours. The reaction solution was poured into ice water and a large amount of light yellow solids precipitated immediately.After filtration, drying and recrystallization from ethyl acetate, 1.06 g of product 3 was obtained as pale yellow crystals. Yield: 80.0% |
| 37% |
|
N-(2-chloropyrimidin-4-yl)-2,3-dimethyl-2H-indazol-6-amine (2.21g; 8mmol, 1 equiv) was dissolved in DMF (11 ml) and cesium carbonate (7.9g; 24.2mmol, 3 equiv) was added.The reaction mixture was stirred for 30 min under nitrogen. Iodomethane (0.8ml, 13mmol,1.6 equiv) was added and the reaction mixture was stirred at room temperature for 2 hrs.The reaction mixture was poured in ice cold water and stirred for 30 minutes. The resulting precipitate was collected by filtration to yield the desired compound (0.84g, 37%). 1H NMR (MeOD, 400MHz) delta= 7.75 (d, J = 7Hz, IH), 7.71 (d, J = 7Hz, IH), 7.35 (d, J =2Hz, IH), 6.8 (dd, J = 2Hz, J = 9Hz, IH), 6.14 (d, J = 6Hz, IH), 4.01 (s, 3H), 3.4 (s, 3H),2.56 (s, 3H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping