Alternatived Products of [ 43210-67-9 ]
Product Details of [ 43210-67-9 ]
| CAS No. : | 43210-67-9 |
MDL No. : | MFCD00144301 |
| Formula : |
C15H13N3O2S
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | HDDSHPAODJUKPD-UHFFFAOYSA-N |
| M.W : |
299.35
|
Pubchem ID : | 3334 |
| Synonyms : |
Methyl 5-(phenylthio)-2-benzimidazolecarbamate
|
Chemical Name : | methyl (6-(phenylthio)-1H-benzo[d]imidazol-2-yl)carbamate |
Application In Synthesis of [ 43210-67-9 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 43210-67-9 ]
- 1
-
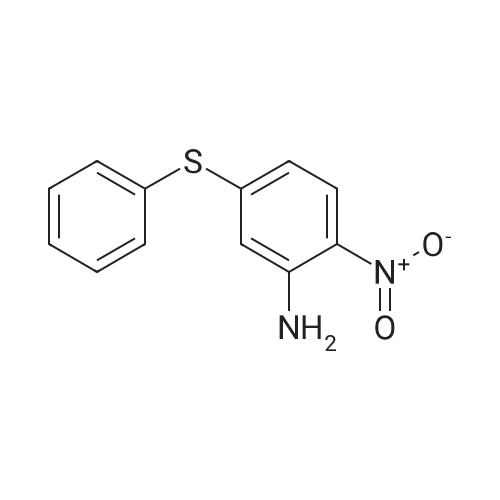 [ 43156-47-4 ]
[ 43156-47-4 ]

-
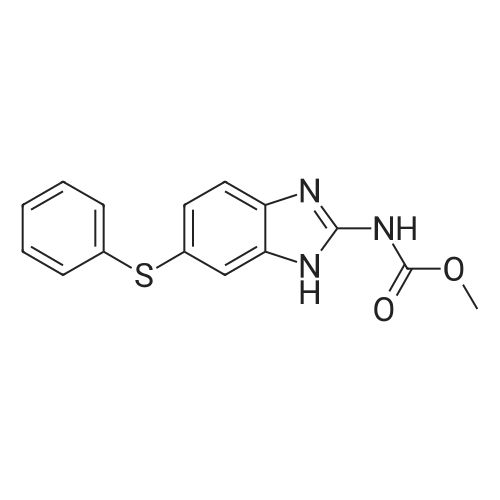 [ 43210-67-9 ]
[ 43210-67-9 ]
- 2
-
 [ 43210-67-9 ]
[ 43210-67-9 ]

-
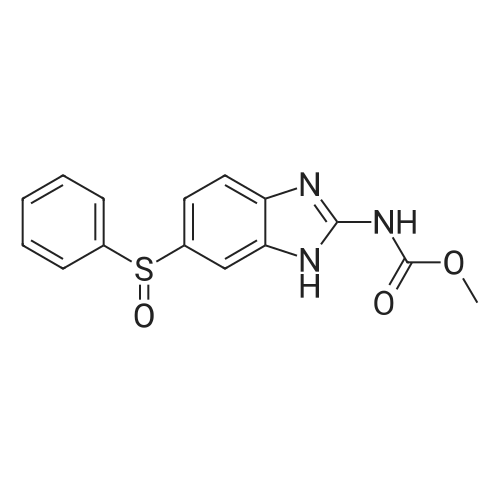 [ 53716-50-0 ]
[ 53716-50-0 ]
| Yield | Reaction Conditions | Operation in experiment |
| 98.8% |
With dihydrogen peroxide; sodium sulfite; In methanol; water; |
EXAMPLE 2 186.4 g of fenbendazole are dissolved in 700 ml of methanol, 513 g of 48% strength hydrobromic acid and 400 ml of water at 60 C., and 65.4 g of a 35% strength hydrogen peroxide solution are added at a pH of -0.5. The reaction solution is heated at 60 C. for 7 hours, and 10.0 g of sodium sulfite are then added. After cooling to room temperature, the reaction mixture is introduced into 20% strength sodium hydroxide solution, the product crystallizing out. After drying in vacuo, 194.5 g of oxfendazole remain, corresponding to a yield of 98.8%, relative to the fenbendazole employed. The purity is 98.7%. |
| 98% |
With urea hydrogen peroxide adduct; In formic acid; water; at 25 - 45℃; for 5.5h; |
(1) Oxidation: 75 g of formic acid and 30 g (0.1 mol) of fenbendazole were added to a 250 mL four-necked flask, and the temperature was raised with stirring, and the temperature was raised to 45 C., so that the reaction solution was completely dissolved.The temperature was lowered to 35 C, 31.7g (0.101mol) of a 30.0% urea peroxide aqueous solution was added dropwise, and the dropwise addition was completed within 3.5 hours, and the dropwise addition temperature was 25-35 C.After dripping and holding for 2 hours, the reaction was stopped by adding 1.2 g of sodium sulfite.(2) Decolorization and crystallization: add 1.2g of activated carbon to the oxidation reaction solution in the previous step, hold at 45-50 C for 1 hour, and then filter with suction. The filtrate is transferred to a 500ml reaction bottle at 45-50 C within 2-3 hours Add 55g of isopropanol, lower the temperature to 20 C, add 70g of 30% liquid alkali dropwise, adjust pH = 5, after the end of the addition, filter with suction, wash with water to pH = 6-7, and dry at 90 C for 15 hours to obtain white Ofendazole 30.9g, yield 98.0%.The content detected by HPLC was 98.9%, impurity A was 0.23%, and impurity B was 0.94%. |
- 3
-
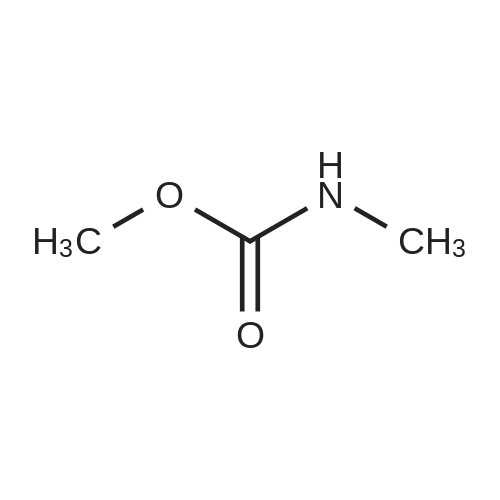 [ 6642-30-4 ]
[ 6642-30-4 ]

-
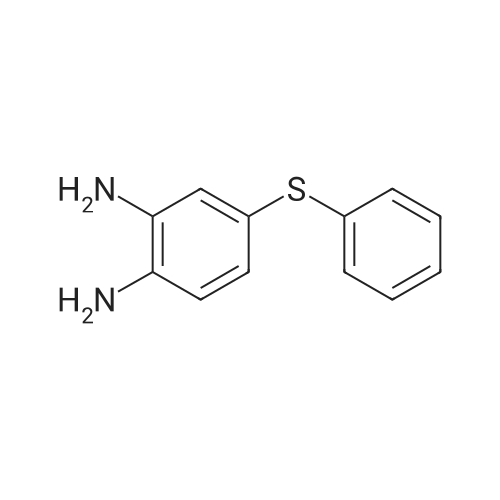 [ 43156-48-5 ]
[ 43156-48-5 ]

-
 [ 43210-67-9 ]
[ 43210-67-9 ]
| Yield | Reaction Conditions | Operation in experiment |
| 86.8% |
With hydrogenchloride; In toluene; at 20 - 55℃; for 2h; |
355.0 g of methyl cyanamidoformate which was cryopreserved in the step 1 was added to a solution of 545.5 g of the intermediate 4 in toluene in the step 5 at room temperature.Raise the temperature to 45-55 C, and add 67.65 g of concentrated hydrochloric acid (36.5%) under stirring. After the addition is completed, stir at 55-60 C for 2 hours.After completion of the reaction, the temperature was lowered to 10-15 C, filtered, and the filter cake was washed with 202.5 g of methanol.The moisture product was dried under hot air at 70 C for 6 hours to obtain 124.5 g of dry product, the purity was 99.7%, and the total yield of the product was 86.8%. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping










