Hierarchical porous activated carbon-supported ruthenium catalysts for catalytic cleavage of lignin model compounds
Pham, Xuan-Tien
;
Tran, Vy Anh
;
Tran, Lan-Trinh Thi
, et al.
Energies,2022,?15(22):8611.
DOI:
10.3390/en15228611
More
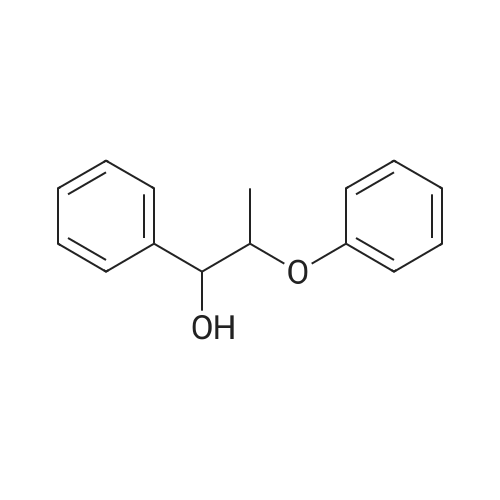
Abstract: The catalytic conversion of lignin model compounds was performed using Ru/C catalysts and an autoclave reactor. The Ru/C catalysts were prepared by the impregnation method using highly porous homemade activated carbon and characterized by XRD, SEM, and specific surface area. The catalytic reactions were performed in a high pressure/temperature reactor at different temperatures and with different solvents. The results showed that the novel Ru/C catalysts prepared from carbon supports activated by the KOH agent showed higher catalytic activity than the commercial catalyst. Ethanol and 2-propanol were suitable solvents for the cleavage of the β–O–4 ether bond of 2-phenoxy1-phenyl ethanol (~65–70% conversion) over a Ru/C-KOH-2 catalyst at 220 ?C in comparison to tert-butanol and 1-propanol solvents (~43–47% conversion of 2-phenoxy-1-phenyl ethanol). Also, the increase in reaction temperature from 200 ?C to 240 ?C enhanced the cleavage of the ether bond with an increase in phenol selectivity from 9.4% to 19.5% and improved the catalytic conversion of 2-phenoxy-1-phenyl ethanol from 46.6% to 98.5% over the Ru/C-KOH-2 catalyst and ethanol solvent. The Ru/C-KOH-2 catalyst showed outstanding conversion (98.5%) of 2-phenoxy-1-phenylethanol at 240 ?C, 1 h, ethanol solvent. This novel hierarchical porous activated carbon-supported ruthenium catalyst (Ru/C-KOH-2) can be applied for the further conversion of the lignin compound.
Keywords:
active carbon ;
biochar ;
Ru/C ;
lignin ;
β-O-4 aryl ether
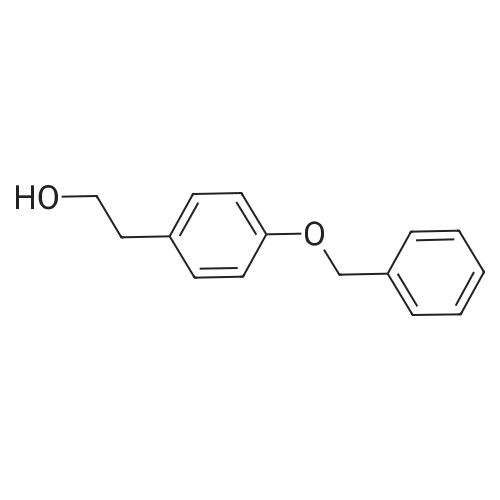
Purchased from AmBeed:
4249-72-3 ;
40515-89-7

Fast screening of Depolymerized Lignin Samples Through 2D‐Liquid Chromatography Mapping
De Saegher, Tibo
;
Lauwaert, Jeroen
;
Vercammen, Joeri
, et al.
ChemistryOpen,2021,10(8):740-747.
DOI:
10.1002/open.202100088
PubMed ID:
34351071
More
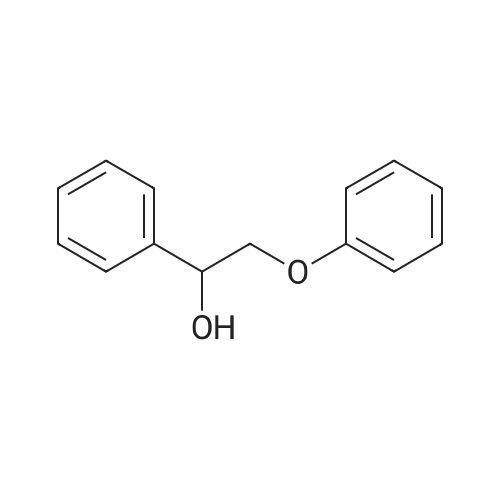
Abstract: Lignin valorization and particularly its depolymerization into bio‐aromatics, has emerged as an important research topic within green chemistry. However, screening of catalysts and reaction conditions within this field is strongly constrained by the lack of analytical techniques that allow for fast and detailed mapping of the product pools. This analytical gap results from the inherent product pool complexity and the focus of the state‐of‐the‐art on monomers and dimers, overlooking the larger oligomers. In this work, this gap is bridged through the development of a quasi‐orthogonal GPC‐HPLC‐UV/VIS method that is able to separate the bio‐aromatics according to molecular weight (hydrodynamic volume) and polarity. The method is evaluated using model compounds and real lignin depolymerization samples. The resulting color plots provide a powerful graphical tool to rapidly assess differences in reaction selectivity towards monomers and dimers as well as to identify differences in the oligomers.
Purchased from AmBeed:
4249-72-3


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping