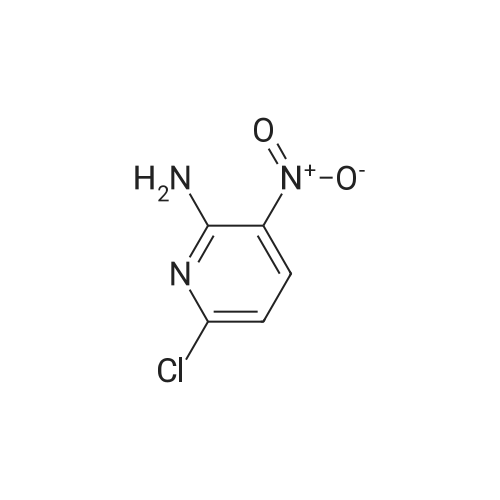
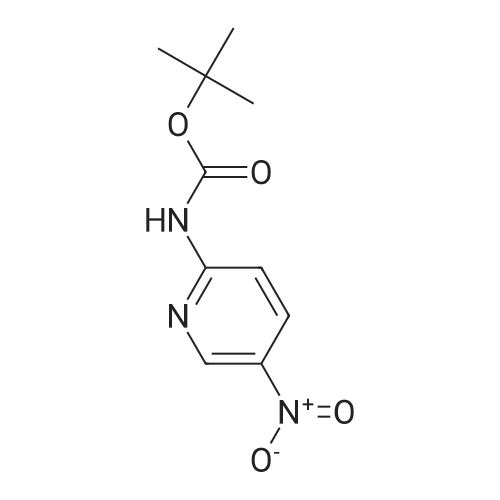
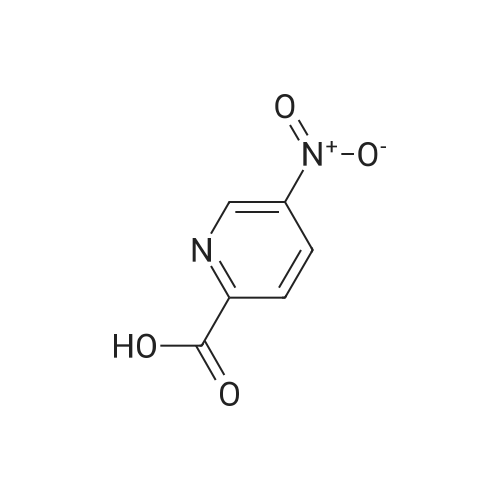
| 62% |
|
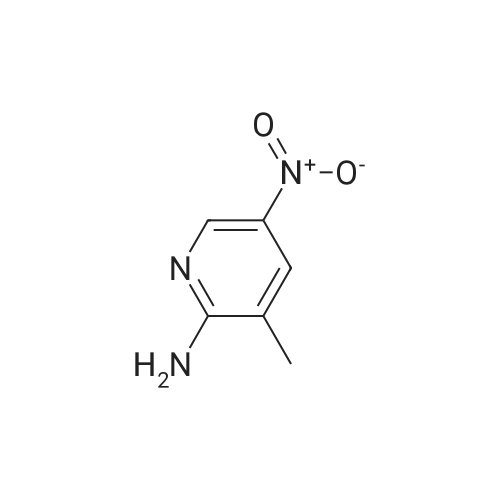
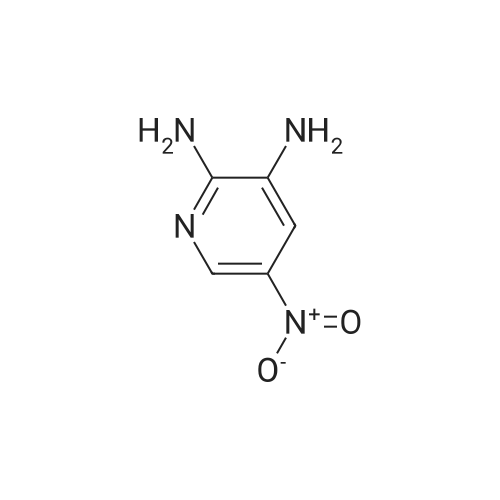
To 0.652 g (4.69 mmol) of 2-amino-5-nitropyridine in THF (5 mL) was added 3.5 mL of NaHMDS (2M solution in THF) at 0 C. After 20 min a solution of 1.085 g (4.97 mmol) of di-tert-butyl dicarbonate in THF (6 mL) was added and the mixture was slowly warmed to room temperature overnight. Water was added, and the mixture was extracted with EtOAc (×4). The organic layer was washed with brine, dried (Na2SO4), and concentrated. Chromatography on, silica with hexanes-EtOAc (7:3), gave 0.695 g (62% yield) of tert-butyl-5-nitropyridin-2-ylcarbamate as an orange powder: 1H NMR (CDCl3) 89.19 (dd, J=2.8, 0.5 Hz, 1H), 8.93 (br s, 1H), 8.46 (ddd, J=9.4, 2.8, 0.5 Hz, 1H), 8.20 (dd, J=9.5, 0.5 Hz, 1H), 1.59 (s, 9H); LCMS (APCI-) m/z: 238 (MH+, 100%). |
| 62% |
|
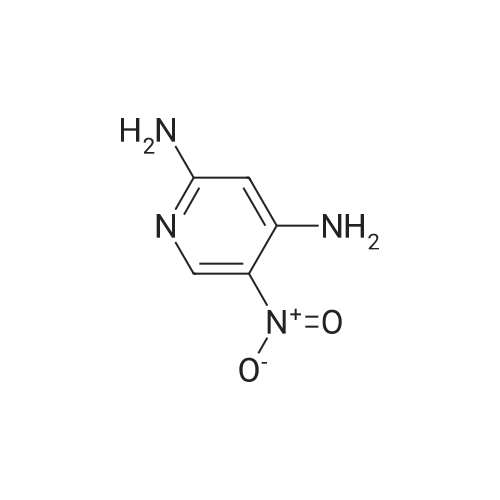
Example 37Synthesis of N5-[4-[2-(difluoromethyl)-4-methoxy-1H-benzimidazol-1-yl]-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2,5-pyridinediamineThe compound was synthesized according to Method A.To 0.652 g (4.69 mmol) of 2-amino-5-nitropyridine in THF (5 mL) was added 3.5 mL of NaHMDS (2M solution in THF) at 0 C. After 20 min a solution of 1.085 g (4.97 mmol) of di-tert-butyl dicarbonate in THF (6 mL) was added and the mixture was slowly warmed to room temperature overnight. Water was added, and the mixture was extracted with EtOAc (×4). The organic layer was washed with brine, dried (Na2SO4), and concentrated. Chromatography on, silica with hexanes-EtOAc (7:3), gave 0.695 g (62% yield) of tert-butyl-5-nitropyridin-2-ylcarbamate as an orange powder: 1H NMR (CDCl3) δ 9.19 (dd, J=2.8, 0.5 Hz, 1H), 8.93 (br s, 1H), 8.46 (ddd, J=9.4, 2.8, 0.5 Hz, 1H), 8.20 (dd, J=9.5, 0.5 Hz, 1H), 1.59 (s, 9H); LCMS (APCI-) m/z: 238 (MH+, 100%).To 0.314 g (1.31 mmol) of the above nitro compound in THF-MeOH (16 mL, 1:1) was added 0.460 g of 10% Pd/C and the mixture was stirred under hydrogen (40 in/Hg) for 4 hrs. The reaction mixture was filtered through celite, washed with MeOH and concentrated to give 0.277 g (99% yield) of tert-butyl 5-aminopyridin-2-yl-carbamate as a white powder: 1H NMR (DMSO-d6) δ9.00 (br s, 1H), 7.62 (dd, J=2.7, 0.4 Hz, 1H), 7.39 (d, J=8.7 Hz, 1H), 6.94 (dd, J=8.7, 2.8 Hz, 1H), 4.92 (s, 2H), 1.44 (s, 9H).To 0.277 g (1.33 mmol) of the above amino compound in THF (3 mL) was added 0.61 mL of n-butyllithium (2.5 M solution in hexanes) and the mixture was stirred for 10 min. A solution of 0.176 g (0.44 mmol) of 1-[4-chloro-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2-(difluoromethyl)-4-methoxy-1H-benzimidazole in THF (5 mL) was added and the resulting mixture was stirred for 1 hr at room temperature. The reaction mixture was neutralized with acetic acid, diluted with water, and extracted with EtOAc. The organic layer was washed with water and aq. NH3, dried, and concentrated. Chromatography on silica, eluting with hexanes-EtOAc (7:3), then with CH2Cl2-EtOAc (3:1), gave 0.033 g (13% yield) of tert-butyl 5-[4-[2-(difluoromethyl)-4-methoxy-1H-benzimidazol-1-yl]-6-(4-morpholinyl)-1,3,5-triazin-2-yl]amino}-2-pyridinylcarbamate: 1H NMR (DMSO-d6) δ10.02 (s, 1H), 9.66 (s, 1H), 8.54 (s, 1H), 8.17-7.80 (m, 4H), 7.39 (d, J=8.7 Hz, 1H), 6.97-6.93 (m, 1H), 3.98 (s, 3H), 3.82 (s, 4H), 3.74-3.72 (m, 4H), 1.48 (s, 9H).To 0.033 g (0.06 mmol) of the above carbamate in CH2Cl2 (3 mL) was added 0.1 mL (1.30 mmol) of trifluoroacetic acid, and the mixture was stirred for 5 hrs. The reaction mixture was diluted with CH2Cl2 and aq. NH4OH, and the organic layer was washed with brine, dried (Na2SO4), and concentrated. The residue was recrystallized from EtOH/CH2Cl2 to give 0.0133 g (49% yield) of N5-[4-[2-(difluoromethyl)-4-methoxy-1H-benzimidazol-1-yl]-6-(4-morpholinyl)-1,3,5-triazin-2-yl]-2,5-pyridinediamine, as a brown powder: mp 267-270 C.; 1H NMR (DMSO-d6) δ9.67-9.49 (m, 1H), 8.18-7.27 (m, 5H), 6.96 (d, J=7.6 Hz, 1H), 6.48 (d, J=8.4 Hz, 1H), 5.87-5.75 (m, 2H), 3.98 (s, 3H), 3.81 (s, 4H), 3.71 (s, 4H); HRMS (ESI) M+H+ Calcd. for C21H22F2N9O2: m/z 470.1859. Found: m/z 470.1867. |
|
With dmap; In dimethyl sulfoxide; at 20℃; for 48h; |
Preparative Example 30aPreparation of ?e^butvK5-armno-4-methylpyridm-2-yl¥2-(fl5'.2^-2-ri-(5-cMoropyrirrudin-2- yl)piperidin-4-vncvclopropyl)ethyl>carbamate.Step 1 : fert-butyl (5-nitropyridin-2-yl')carbamate.2-Amino-5-nitropyridine (Ig, 7.2mmol) and di-tert-buty dicarbonate (2.4g, 10.8ramol) were added in DMSO (1OmL), DMAP (170mg, 1.4mmol) was added and stirred at room temperature for 2 days. Water (5OmL) was added, extracted with ethyl acetate (10OmL), second wash with Brine (5OmL). The organic phase was dried by magnesium sulfate, filtered, concentrated and purified by column chromatography through a 50 gram Biotage SNAP KP-Sil silica gel cartridge eluting with 13% ethyl acetate/hexanes to give the title compound as a white solid. LRMS calc: 239.23; obs: 262.34 (M+23), 184.14 (M-55), 166.11 (M-73). |
|
With lithium hexamethyldisilazane; In tetrahydrofuran; at 0 - 20℃; |
PREPARATION 46 tert-Butyl 5-nitropyridin-2-ylcarbamate 200 mL of THF was added to 5-nitropyridin-2-amine (5g, 35.90 mmol). The solution was cooled to 0 C. Sodium hexamethyldisilazide (NaHMDS, 13.18 g, 71.9 mmol) in 30 mL THF was added dropwise. At the same time, di-tert-butyl dicarbonate (8.3 mL, 35.90 mmol) in 30 mL THF was added dropwise. The reaction mixture was stirred at 0 C for 15min and then warmed to room temperature. The reaction mixture stopped stirring due to salt/product formation and was left at room temperature for 20h. 150 mL 0.5N HCl was added and 150 mL ethyl acetate was added. The layers were separated. The organic layer was washed with 50 mL brine, dried with Na2SO4, filtered and evaporated to dryness. A yellow solid was isolated, which was used in the next synthetic step without further purification. LRMS (m/z): 240 (M+1)+ |
|
With sodium hexamethyldisilazane; In tetrahydrofuran; at 0 - 20℃; for 0.25h; |
200 mL of THF was added to 5-nitropyridin-2-amine (5g, 35.90 mmol). The solution was cooled to 0 C. Sodium hexamethyldisilazide (NaHMDS, 13.18 g, 71 .9 mmol) in 30 mL THF was added dropwise. At the same time, di-ie f-butyl dicarbonate (8.3 mL, 35.90 mmol) in 30 mL THF was added dropwise. The reaction mixture was stirred at 0 C for 15min and then warmed to room temperature. The reaction mixture stopped stirring due to salt/product formation and was left at room temperature for 20h. 150 mL 0.5N HCI was added and 150 mL ethyl acetate was added. The layers were separated. The organic layer was washed with 50 mL brine, dried with Na2S04, filtered and evaporated to dryness. A yellow solid was isolated, which was used in the next synthetic step without further purification.LRMS (m/z): 240 (M+1 )+ |
| 94.6 g |
With dmap; triethylamine; In dichloromethane; at 20℃; for 18h; |
A 3-L round-bottomed flask was charged with 5-nitro-2-pyridinamine (75.0 g, 539 mmol, Alfa Aesar, Ward Hill, MA) and 500 mL of DCM. To this was added triethylamine (82 g, 809 mmol), di-tert-butyl dicarbonate (129 g, 593 mmol, Sigma-Aldrich, St. Louis, MO), and DMAP (32.9 g, 270 mmol, Sigma- Aldrich, St. Louis, MO). After stirring at rt for 18 h, the mixture was diluted with water and the solid was collected by filtration. The yellow solid was washed with MeOH to give tert-butyl (5-nitro-2-pyridinyl)carbamate (94.6 g) as a slightly-yellow solid. |
| 94.6 g |
With dmap; triethylamine; In dichloromethane; at 20℃; for 18h; |
A 3-L round-bottomed flask was charged with 5-nitro-2-pyridinamine (75.0 g, 539 mmol, Alfa Aesar, Ward Hill, MA) and 500 mL of DCM. To this was added triethylamine (82 g, 810 mmol), di-tert-butyl dicarbonate (129 g, 593 mmol, Sigma-Aldrich, St. Louis, MO), and N,N-dimethylpyridin-4-amine (32.9 g, 270 mmol, Sigma-Aldrich, St. Louis, MO). After stirring at rt for 18 h, the mixture was diluted with water and the solid was collected by filtration. The yellow solid was washed with MeOH to give tert-butyl (5-nitro-2- pyridinyl)carbamate (94.6 g) as a light yellow solid. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping