|
With dibenzyl azodicarboxylate; triphenylphosphine; In tetrahydrofuran; at 20℃;Product distribution / selectivity; |
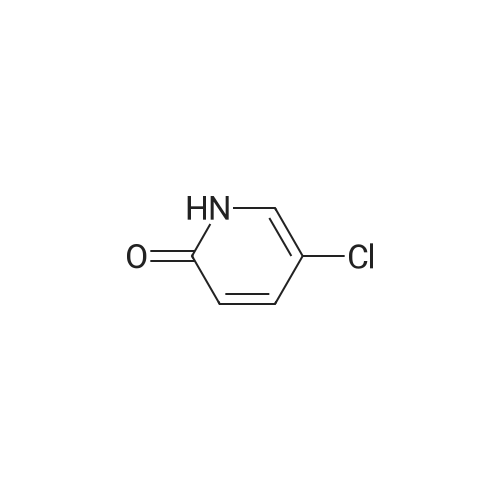
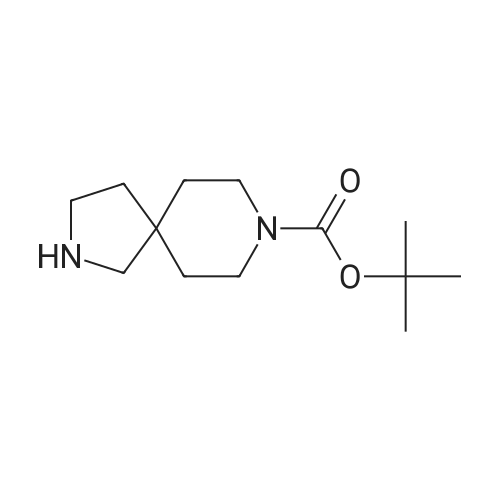
In a flame-dried flask under N2, was combined 807 mg (6.24 mmol, 1.33 eq) of 5- chloro 2-pyridyl phenol and 1.64 g (6.23 mmol, 1.33 eq) of triphenylphosphine in 20 mL of anhydrous THF at room temperature. To this solution was added 1.86 g (6.233 mmol, 1.33 eq) of DBAD, followed by 1.0 g (4.69 mmol, 1.0 eq) of 6-hydroxymethyl-3-aza- <n="58"/>bicyclo[3.1.0]hexane-3-carboxylic acid tert-butyl ester in 5 ml_ of anhydrous THF. The yellow solution was stirred at room temperature overnight. To this solution was added 6.3 mL (25.2 mmol, 5.4 eq) of 4M HCI in 1 ,4-dioxane and the resulting solution was stirred at room temperature overnight. The solution was concentrated under reduced pressure and the resulting residue was dissolved in 95 mL methylene chloride. The solution was extracted with 63 mL of 15percent aqueous citric acid. The aqueous layer was extracted with 95 mL of methylene chloride. The aqueous layer was basified with 32 mL of cone. NH4OH and extracted three times with 65 mL of methylene chloride. The combined organic layers were dried over anhydrous MgSO4, filtered and stripped in vacuo to give yellow oil which crystallized while drying under high vaccum (588 mg). The crude material was purified via flash chromatography, eluting with 5percent methanol/ methylene chloride- with 0.1 percent triethyl amine to yield 242 mg of desired compound. 400 MHz 1H NMR (CDCI3) delta 8.0 (d, 1 H), 7.5 (m, 1H), 6.7 (d, 1 H), 4.1 (d, 2H), 3.1 (d, 2H), 2.9-3.0 (m, 3H), 1.5 (m, 2H), 1.2 (m, 1H); MS (M+1) 225, 227. |
|
With triphenylphosphine; diethylazodicarboxylate; In tetrahydrofuran; at 20℃; for 64h;Product distribution / selectivity; |
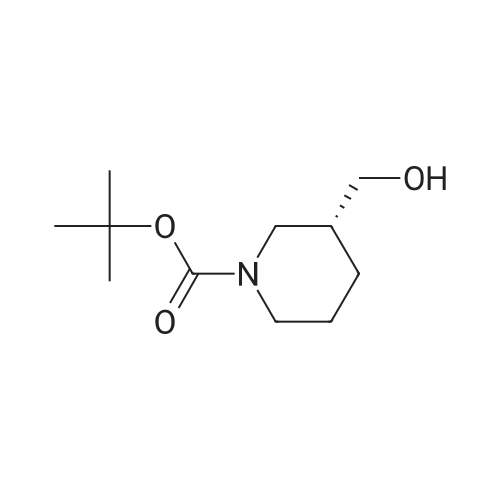
19.5 mmol of triphenyl phosphine was dissolved in 9.75 mL of anhydrous THF and vortexed. 75 mul (0.15 mmol) of this solution was added to each 0.1 mmol of varying phenols of general foumula (III) in 2-dram vials. 13.0 mmol of (1S,5R,6R)-tert-butyl 6-(hydroxymethyl)-3- <n="59"/>aza-bicycio[3.1.0]hexane-3-carboxylate (Preparation 1 ) was dissolved in 6.5 mL of anhydrous THF and vortexed. 50 muL (0.10 mmol) of this solution was added to each vial. 19.5 mmol of Diethylazodicarboxylate (DEAD) was dissolved in 9.75 mL of anhydrous THF and vortexed. 75 muL (0.15 mmol) of this solution was added to each vial. The vials were sealed and placed on shaker at room temperature for 64 hours. 200 muL of 4 M HCI in 1 ,4-dioxane was then added to each vial and the vials were placed on a shaker at room temperature for 16 hours. The reactions were evaporated under nitrogen. MCX SPE columns were conditioned by eluting twice with 4 mL of CH3OH. The reaction residues were dissolved with 1.0 mL of CH3OH, vortexed, and the reaction residues were added to the MCX columns. The reaction vials were rinsed two times with 1.0 mL of CH3OH, vortexed, and the reaction residues were added to the columns. The columns were eluted three times with 4.0 mL of CH3OH. 4.0 mL of 2 M NH3 in CH3OH was added to each vial with the eluent collected in tared 2-dram vials. The vials were evaporated under nitrogen, tared, and used without further purification in the next reaction. To each of the vials prepared above were added 500 muL of 1 ,2-dichloroethane and 28 muL (0.2 mmol) of triethylamine. 4.3 mmol of an aldehyde of general formula (V) was dissolved in 4.3 mL of 1 ,2-dichloroethane and 0.1 mL (0.1 mmol) was added to the vials. 0.3 mmol of sodium triacetoxyborohydride was added to vials. The vials were sealed and placed on shaker at room temperature for 16 hours. 1.5 mL of 1 N NaOH, followed by 1.0 mL of 1 ,2- dichloroethane was added to each vial and vortexed for 4 minutes. The layers were allowed to separate and 1.5 mL was removed from the lower layer to empty SPE barrels, which were allowed to drip into tared 2-dram vials. 1.0 mL of 1 ,2-dichloroethane was added and the mixture was vortexed for 4 minutes. The layers were allowed to separate and 1 mL was removed from the lower layer to empty SPE barrels, which were allowed to drip into the tared 2-dram vials. 1.0 mL of 1 ,2-dichloroethane was added to the each vial and vortexed for 4 minutes. The layers were allowed to separate and 1 mL was removed from the lower layer to empty SPE barrels, which were allowed to drip into tared 2-dram vials. The combined SPE eluents remaining in the 2 dram tared vials were evaporated in a Savant SpeedVac Plus. The resulting crude mixtures were purified via preparative LC/MS chromatography to yield compounds of general formula (Vl).Alternatively, compounds of formula I can be prepared as highlighted below in method C, utilizing parallel chemistry or high-speed synthesis methods. |
|
With triphenylphosphine on polystyrene; diethylazodicarboxylate; In tetrahydrofuran; toluene; at 20℃; for 17h;Product distribution / selectivity; |
Prepared a 2 M stock solution of (1S,5R,6R)-tert-butyl 6-(hydroxymethyl)-3-aza- bicyclo[3.1.0]hexane-3-carboxyiate (Preparation 1 ) in THF. Prepared 2 M stock solutions of varying phenols of a general formula (III) <n="60"/>in THF. Prepared a 0.5 M stock solution of DEAD in toluene. To each reaction vial, added 0.200 ml_ of the varying phenol followed by 0.075 mL of (1S,5R,6R)-tert-butyl 6- (hydroxymethyl)-3-aza-bicyclo[3.1.0]hexane-3-carboxylate. Added 0.600 mL of the DEAD solution, followed by 0.750 mL of toluene. Added 140 mg of triphenylphosphine-polystyrene resin. Vials were capped and shaken at room temperature for 17 hours. Added 2.5 mL of THF to each reaction vial. The top layer was transferred to empty 6 mL SPE cartridges over collection tubes. Added 3.0 mL of THF to the reaction vials and then aspirated the top layer to the SPE cartridges over collection tubes. Transferred solutions from collection tubes to new reaction vials and evaporated. Added 0.600 mL of CH3OH followed by 0.300 mL of 4 M HCI in 1 ,4-dioxane to each reaction vial. Vials were capped and shaken at room temperature for 24 hours. The solvent was evaporated and the intermediates used without further purification in the next reaction.Prepared a 0.25 M solution of an aldehyde of general formula (V) in 1 ,2- dichloroethane. Prepared a 0.25 M solution of sodium triacetoxyborohydride in 1 ,2- dichloroethane. Added 0.600 mL of the aldehyde solution to each of the reaction vials from the step above, followed by 0.070 mL of DIPEA. Added 2.0 mL of the sodium triacetoxyborohydride solution, capped vials and shaken at room temperature for 17 hours. Added 1.0 mL of 1 ,2-dichloroethane followed by 2.0 mL of 10percent NaOH. Vials were vortexed and/or shaken and removed top layer. Added 2.0 mL of 10percent NH4OH, with vials shaken well and/ or vortexed. The bottom layers were aspirated to empty 6 mL SPE cartridges over tared collection tubes. Added 1.0 mL of 1 ,2-dichloroethane to the aqueous layer and aspirated the bottom layer to the SPE cartridge over the collection tubes. Evaporated solutions to dryness. The resulting crude mixtures were purified via preparative LC/MS chromatography to yield compounds of general formula (Vl) Alternatively, compounds of formula I can be prepared as highlighted below in method D utilizing parallel chemistry or high-speed synthesis methods. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping