|
With N-ethyl-N,N-diisopropylamine; HATU; In N,N-dimethyl-formamide; at 20℃; for 0.5h;Product distribution / selectivity; |
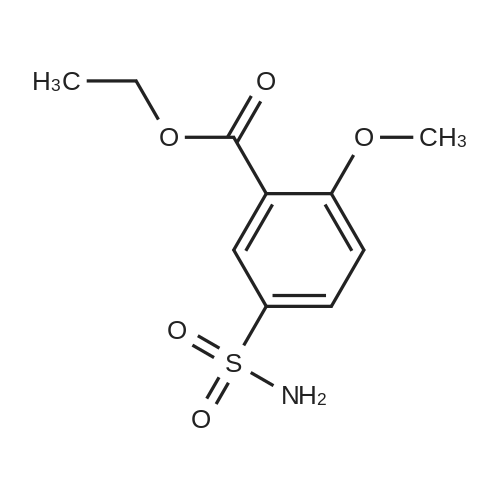
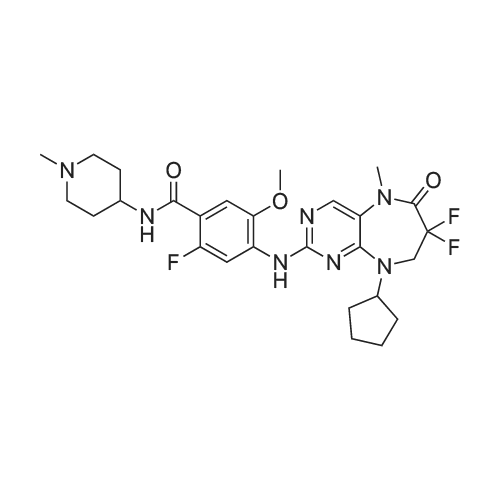
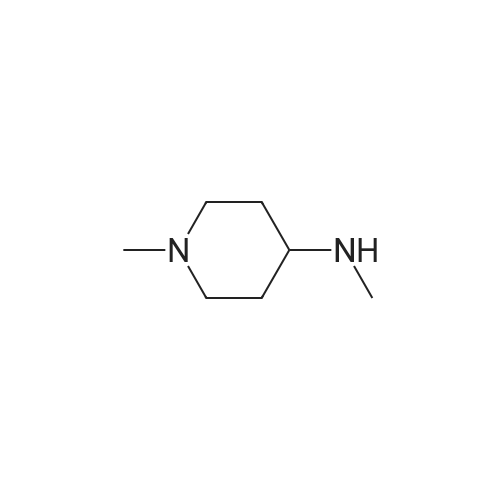
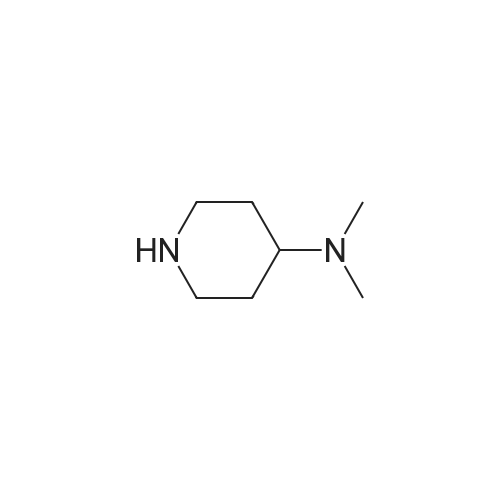
Compound 116: 4-(9-Cyclopentyl-7,7-difluoro-5-methyl-6-oxo-6,7,8,9- tetrahydro-5H-pyrimido[4,5-b][l,4]diazepin-2-ylamino)-2-fluoro-5-methoxy-N-(l- methylpiperidin-4-yl)benzamide; The title compound was synthesized from 4-(9-cyclopentyl-7,7-difluoro-5-methyl-6-oxo- 6,7,8,9-tetrahydro-5H-pyrimido[4,5-b][l,4]diazepin-2-ylamino)-2-fluoro-5- <n="360"/>methoxybenzoic acid and l-methylpiperidin-4-amine as described in the general procedure for amide bond synthesis. The final compound was purified by reverse phase HPLC and basified to give the free base. 1U NMR (400 MHz, DMSO-J6) delta ppm 1.60-1.73 (m, 11 H), 1.88 - 2.21 (m, 8 H) 2.78 (br. s., 2 H) 3.73 (br. s., 1 H) 3.91 (s, 3 H) 4.08 (t, J=13.8 Hz, 2 H) 4.81 (d, J=8.1 Hz, 1 H) 7.18 (d, J=6.6 Hz, 1 H) 7.91 (br. s., 1 H) 8.04 (s, 1 H) 8.24 (d, J=13.4 Hz, 1 H) 8.30 (s, 1 H). [M+H] calc'd for C27H34F3N7O3, 562; found 562.; The title compound was synthesized from 4-(9-cyclopentyl-7,7-difluoro-5- methyl-6-oxo-6,7,8,9-tetrahydro-5H-pyrimido[5,4-b][l,4]diazepin-2-ylamino)-2-fluoro-5- methoxybenzoic acid (obtained above, 12 mg) as described in the general procedure for amide bond synthesis using HATU and l-methylpiperidin-4-amine. The final compound was purified by reverse phase HPLC and basified to give the free base (8 mg, 55percent). 1H NMR (400 MHz, DMSO-J6) delta ppm 1.60-1.73 (m, 11 H), 1.88 - 2.21 (m, 8 H) 2.78 (br. s., 2 H) 3.73 (br. s., 1 H) 3.91 (s, 3 H) 4.08 (t, J= 14 Hz, 2 H) 4.81 (d, J= 8 Hz, 1 H) 7.18 (d, J= 7 Hz, 1 H) 7.91 (br. s., 1 H) 8.04 (s, 1 H) 8.24 (d, J= 13 Hz, 1 H) 8.30 (s, 1 H). [M+H] calc'd for C27H34F3N7O3, 562; found 562. |
|
|
(I-176) To a mixture of 0.05 g (0.11 mmole) of 4-(9-cyclopentyl-7,7-difluoro-5-methyl-6-oxo-6,7,8,9-tetrahydro-5H-pyrimido[4,5-b][1,4]diazepin-2-ylamino)-2-fluoro-5-methoxy-benzoic acid (I-169), 0.06 g (0.16 mmole) of 1-[bis(dimethylamino)methylene]-1H-benzotriazolium-3-oxide hexafluorophosphate and 2 mL of dimethylformamide was added 0.06 mL (0.32 mmole) of diisopropylethylamine. After stirring at room temperature for 30 minutes 0.04 g (0.32 mmole) of 4-amino-1-methylpiperidine was added. The mixture was stirred at room temperature for 18 hours, then diluted with ethyl acetate and water. The aqueous phase was extracted twice with ethyl acetate. The combined organic layers were washed with 1M sodium hydroxide solution then brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel chromatography eluding with methanol-dichloromethane (gradient, 0:100-20:80) to give 0.038 g of 4-(9-cyclopentyl-7,7-difluoro-5-methyl-6-oxo-6,7,8,9-tetrahydro-5H-pyrimido[4,5-b][1,4]diazepin-2-ylamino)-2-fluoro-5-methoxy-N-(1-methyl-piperidin-4-yl)-benzamide (I-176). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 HazMat Fee +
HazMat Fee +

 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping