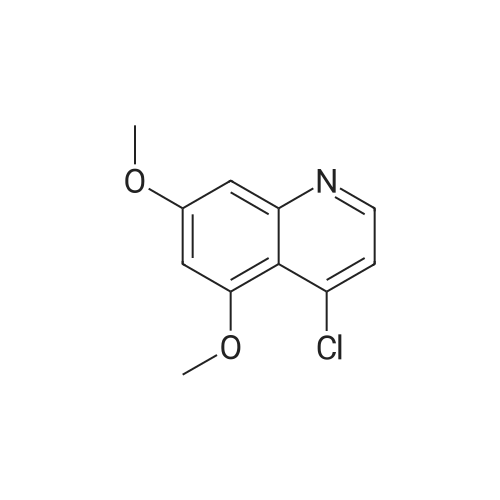
| 96.3% |
With potassium tert-butylate; In dimethyl sulfoxide; at 20 - 65℃; for 22h;Product distribution / selectivity; |
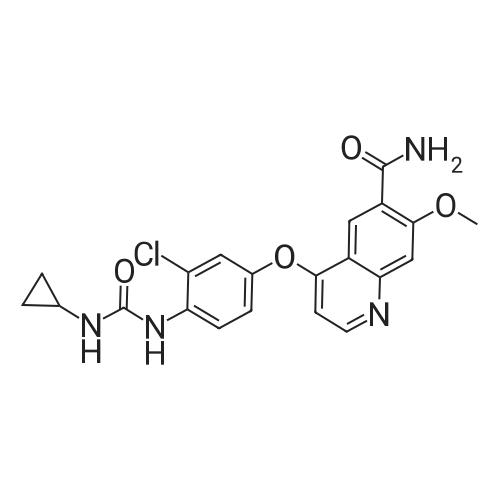
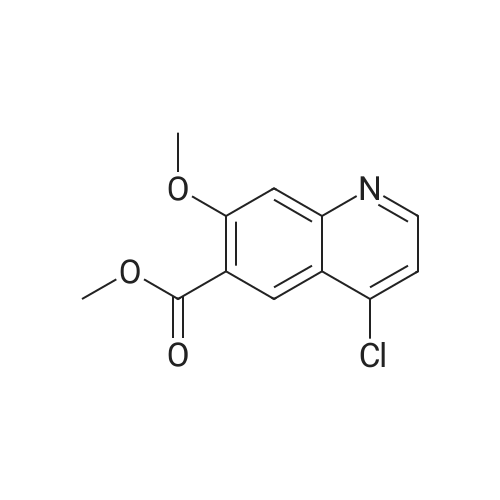
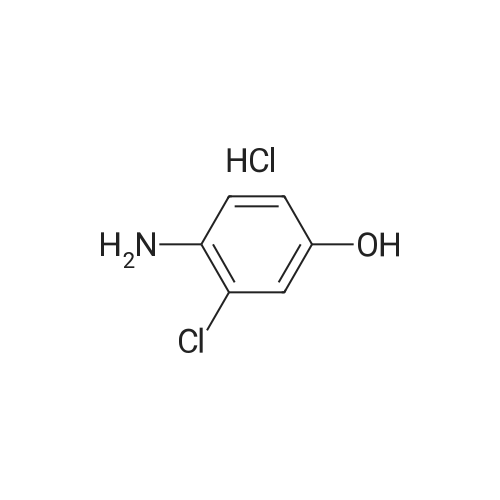
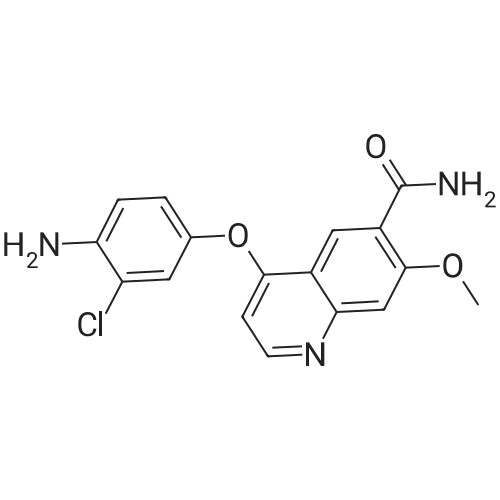
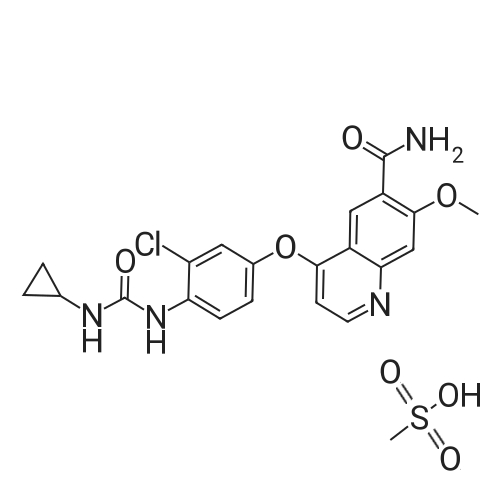
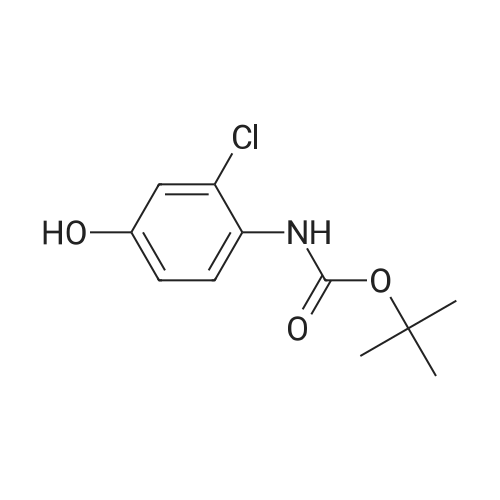
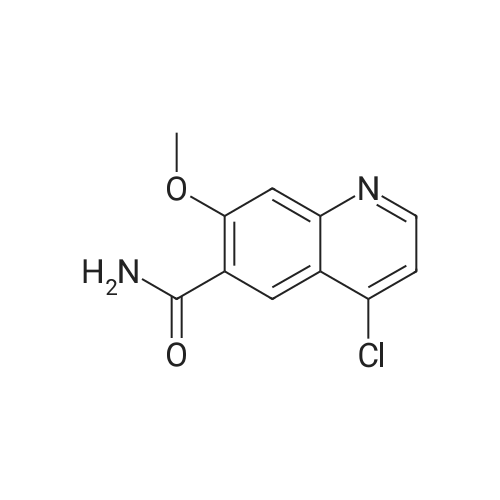
(Preparation Example 3) Preparation (3) of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide 7-Methoxy-4-chloroquinoline-6-carboxamide (5.00 kg, 21.13 mol), dimethyl sulfoxide (55.05 kg), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (5.75 kg, 25.35 mol) and potassium t-butoxide (2.85 kg, 25.35 mol) were introduced in this order into a reaction vessel under a nitrogen atmosphere. The mixture was stirred for 30 min at 20 C, and the temperature was raised to 65 C over 2.5 hours. The mixture was stirred at the same temperature for 19 hours. 33% (v/v) acetone-water (5.0 L) and water (10.0 L) were added dropwise over 3.5 hours. After the addition was completed, the mixture was stirred at 60 C for 2 hours. 33% (v/v) acetone-water (20.0 L) and water (40.0 L) were added dropwise at 55 C or more over 1 hour. After stirring at 40 C for 16 hours, precipitated crystals were collected by filtration using a nitrogen pressure filter, and was washed with 33% (v/v) acetone-water (33.3 L), water (66.7 L), and acetone (50.0 L) in that order. The obtained crystals were dried at 60 C for 22 hours using a conical vacuum dryer to give 7.78 kg of the titled compound (yield: 96.3%). Further, all of the 1H-NMR chemical sift values of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide prepared in Preparation Examples 1 to 3 described above agreed with those of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide described in. |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; for 23h;Product distribution / selectivity; |
To dimethylsulfoxide (20 mL) were added 7-methoxy-4-chloro-quinoline-6-carboxamide (0.983 g), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), followed by heating and stirring at 70C for 23 hours. After the reaction mixture was allowed to cool down to room temperature, water (50 mL) was added, and the produced crystals were collected by filtration to give 1.56 g of the title compound (88% yield). 1H-NMR(d6-DMSO): 0.41(2H, m), 0.66(2H, m), 2.56(1H, m), 4.01(3H, s), 6.51(1H, d, J=5.6Hz), 7.18(1H, d, J=2.8Hz), 7.23(1H, dd, J=2.8, 8.8Hz), 7.48(1H, d, J=2.8Hz), 7.50(1H, s), 7.72(1H, s), 7.84(1H, s), 7.97(1H, s), 8.25(1H, d, J=8.8Hz), 8.64(1H, s), 8.65(1H, d, J=5.6Hz) |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; for 23h;Product distribution / selectivity; |
(3) Preparation of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide ; To dimethyl sulfoxide (20 mL) were added <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (0.983 g), ;1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), and the mixture was heated and stirred at 70 C for 23 hours. The reaction mixture was cooled to room temperature, and water (50 mL) was added, and the resultant crystals were then filtered off to give 1.56 g of the titled compound (yield: 88%). |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; for 23h;Product distribution / selectivity; |
(3) Preparation of 4-(3-chloro-4-5 (cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide To dimethylsulfoxide (20 mL) were added <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (0.983 g), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), followed by heating and stirring at 70 C. for 23 hours. After the reaction mixture was allowed to cool down to room temperature, water (50 mL) was added, and the produced crystals were collected by filtration to give 1.56 g of the titled compound (88% yield). |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; for 23h; |
To DMSO (20 mL) were added 7-methoxy-4-chloro-quinoline-6-carboxamide (0.983 g), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), and the mixture was heated and stirred at 70 C. for 23 hours. The reaction mixture was cooled to room temperature, water (50 mL) was added, and the resultant solid was then filtered off to give 1.56 g of the titled compound (yield: 88%). 1H-NMR (d6-DMSO): 0.41 (2H, m), 0.66 (2H, m), 2.56 (1H, m), 4.01 (3H, s), 6.51 (1H, d, J=5.6 Hz), 7.18 (1H, d, J=2.8 Hz), 7.23 (1H, dd, J=2.8, 8.8 Hz), 7.48 (1H, d, J=2.8 Hz), 7.50 (1H, s), 7.72 (1H, s), 7.84 (1H, s), 7.97 (1H, s), 8.25 (1H, d, J=8.8 Hz), 8.64 (1H, s), 8.65 (1H, d, J=5.6 Hz). |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; for 23h;Product distribution / selectivity; |
(3) Preparation of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide To dimethyl sulfoxide (20 mL) were added <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (0.983 g), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), and the mixture was heated and stirred at 70 C for 23 hours. The reaction mixture was cooled to room temperature, and water (50 mL) was added, and the resultant crystals were then collected by filtration to give 1.56 g of the titled compound (yield: 88%). |
| 88% |
With caesium carbonate; In dimethyl sulfoxide; |
(2) production of 4-(3-Chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide TO dimethylsulfoxide (20 mL) were added <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (0.983 g), 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (1.13 g) and cesium carbonate (2.71 g), and the mixture was stirred at 70 C. for 23 hours. The mixture was cooled to room temperature, addition of water (50 mL) yielded crystals, and the crystals were filtered off to give the title compound (1.56 g, yield 88%). |
| 86.6% |
With caesium carbonate; In dimethyl sulfoxide; at 85℃; for 12h; |
Into a 250 ml round bottom three-mouth flask, add <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (2.0g, 8 . 4mmol), compound IV (1.9g, 8.4mmol), cesium carbonate (5.5g, 16.9mmol) and dimethylsulfoxide (40 ml). At 85 C heating and stirring 12 hours. After cooling to room temperature the reaction solution, the addition of water (100 ml), filtering of the crystalline compound 3.1g, the yield of 86.6%. |
| 86.2% |
With caesium carbonate; In dimethyl sulfoxide; at 70℃; |
1-(2-Chloro-4-hydroxyphenyl)-3-cyclopropylurea prepared according to steps (1) and (2) of Example 1 was weighed.Compound E) 34.0 g (150 mmol), 4-chloro-7-methoxyquinolin-6-amide (Compound F) 29.6 g (125 mmol),Barium carbonate81.5 g (250 mmol) in a 1.0 L round bottom flask,Add 600 mL of DMSO, and heat the reaction at 70 C with stirring.The reaction was monitored by TLC. After the reaction is completed, it is cooled to room temperature.Add 1200mL water and stir.Gradual solids are formed in the system, and the mixture is filtered.The filter cake was washed with water to obtain a crude product.The crude product was beaten with an aqueous acetone solution (6/18 = acetone (v) / water (v)).After drying by filtration, a total of 46.0 g of solid product levastatin can be obtained.The yield is 86.2%.Purity >99%, HPLC detection results (purity 99.0%), As shown in Figure 2 (HPLC instrument model is Ultimate 3000; column temperature: 25 C; detection wavelength: 252 nm; column is:phenomenex-C18 4.6 x 250 mm column; mobile phase: acetonitrile: water = 20:80, plus 0.1% formic acid; flow rate: 0.6 ml/min). |
| 82.8% |
With potassium carbonate; In dimethyl sulfoxide; tert-butyl alcohol; at 20 - 70℃; for 8h; |
Weigh 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropyl urea (8) 3g (13mmol),Add 15 mL of dimethyl sulfoxide,1.6 g (14.3 mmol) of tert-butanol, stirred at room temperature for 1 h.The temperature was raised to 70 C, and 1.54 g (6.5 mmol) of 4-chloro-7-methoxyquinolin-6-amide (6) and 0.898 g (8.5 mmol) of potassium carbonate were added thereto, and the mixture was maintained at a temperature of 70 C for 8 hours. After cooling to room temperature, 15 mL of water was added, filtered, and the filter cake was washed several times with water and dried. The obtained crude product was suspended in an aqueous acetone solution of 33% by volume, stirred at 60 C overnight, cooled, and filtered to give a white solid (1) 2.304 g, yield 82.8%, mp 226 to 228 C. The purity of the HPLC was 99.50%. |
| 51.9% |
With caesium carbonate; In N,N-dimethyl-formamide; at 60℃; for 26h; |
Add 15.0 L of N,N-dimethylformamide to the reaction kettle.730.0 g (3.08 mol) of <strong>[417721-36-9]4-chloro-7-methoxyquinolin-6-carboxamide</strong> was added in sequence with stirring.837.0g (3.7mol)1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea and 2.0 kg (6.16 mol) of cesium carbonate,The reaction was stirred at 60 C for 26 h, and the reaction was completed by TLC. Stir and cool, and flush the reaction solution to an appropriate amount of water.The crude lenvatinib was filtered, and the content of the compound of the impurity formula I was determined by HPLC to be 0.32%.27.0 L of methanol was added to the reaction vessel, heated to reflux, and then stirred,The upper step wet product was added to the kettle, dissolved under reflux, and stirred for crystallization. Filtration, the resulting solid was 1.85 g,The yield was 51.9%, and the purity was 98.3%, wherein the content of the compound of the impurity formula I was 0.04%. |
|
|
Firstly, 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea was obtained in a similar manner as Preparation Example 1, and 7-methoxy-4-chloro-quinoline-6-carboxamide was obtained in a similar manner as Preparation Example 2. Then, to a mixture of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (114.9 g), 7-methoxy-4-chloro-quinoline-6-carboxamide (80.0 g) and potassium t-butoxide (56.9 g) was added DMSO (800 mL) at room temperature, and the mixture was heated and stirred at 55 C. for 20 hours and, then further at 60 C. for 4 hours. To the reaction mixture, 33% (v/v) acetone-water (165 mL) was added in 1 minute at 60 C. with stirring. Additional 33% (v/v) acetone water (1035 mL) was added dropwise over 7 minutes to allow the crystals to appear, followed by stirring at 40 C. for 19 hours. The crystals were filtered off, washed with 33% (v/v) acetone-water and acetone, and dried to give 131.9 g of yellowish brown granular crystal (the polymorph (A)).; Examples 1b, 1c and 1d The polymorph (A) of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide was obtained in a similar manner as Example 1a. |
|
|
Firstly, 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea was obtained in a similar manner as Preparation Example 1, and 7-methoxy-4-chloro-quinoline-6-carboxamide was obtained in a similar manner as Preparation Example 2. Secondly, to a mixture of 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea (11.49 g), <strong>[417721-36-9]7-methoxy-4-chloroquinoline-6-carboxamide</strong> (8.00 g) and potassium t-butoxide (5.69 g) was added DMSO (80 mL) at room temperature, and the mixture was heated and stirred at 60 C. for 25 hours. The reaction mixture was divided into four equal parts. To an aliquot was added dropwise 33% (v/v) acetone-water (10 mL) over 3 hours at 60 C. with stirring to allow the crystals to appear. Additional 33% (v/v) acetone-water (20 mL) was added dropwise over 1 hour, and the stirring was continued at 40 C. for 5 hours. The resultant crystals were filtered off, washed with 33% (v/v) acetone-water and acetone, and dried to give 3.22 g of white fibrous crystals (the polymorph (B)).; Examples 2b, 2c and 2d A polymorph (B) of 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide was obtained in a similar manner as Example 2a. |
| 4.1 g |
With sodium methylate; In chloroform; for 6h;Reflux; |
Take 6-carboxamido-7-methoxy-4-chloroquinoline 2.65 g, 1-(2-chloro-4-hydroxyphenyl)-3-cyclopropylurea 2.3 g, 20 mL of a 20% sodium methoxide solution, 20 mL of chloroform, and refluxing reaction for 6 hours. After completion of the reaction, the same procedure as in Example 1 gave a white solid (4.1 g). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping