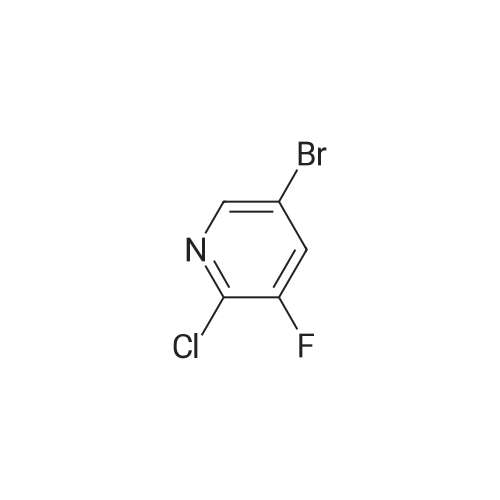
| 89% |
Stage #1: With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78 - -70℃; for 1 h;
Stage #2: With hexachloroethane In tetrahydrofuran; hexane at -78 - 20℃; |
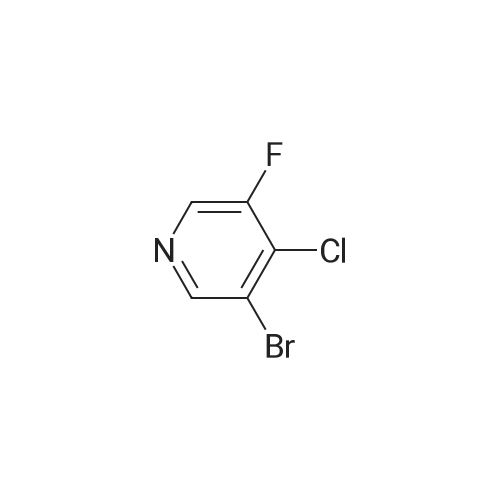
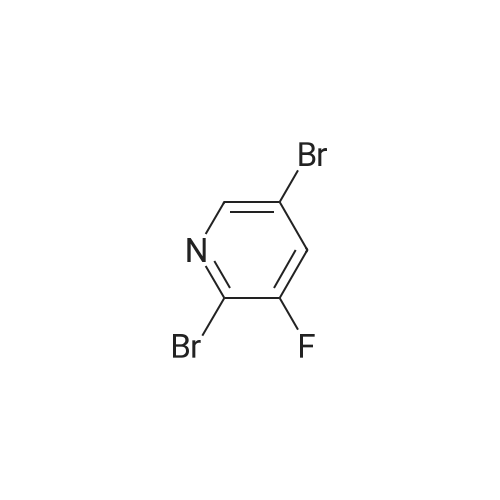
To a solution of diisopropylamine (6.899 g, 9.555 mL, 68.18 mmol) in THF (75 mL) cooled to -78°C, was added butyllithium (25 mL of 2.5 M in hexanes, 62.5 mmol). The reaction mixture was allowed to warm to -20°C then cooled back down to -78°C. A solution of 3-bromo-5-fluoro-pyridine (10 g, 56.82 mmol) in THF (25 mL) was added dropwise keeping temperature below -70°C (approx 30 mins). The reaction mixture was stirred at - 78°C for 30 min and a solution of 1, 1, 1,2,2,2-hexachloroethane (14.8 g, 62.5 mmol) in THF (20 niL) was then added dropwise, keeping temperature below -70°C (over approx 30 mins). The mixture was stirred at -78°C for 20 minutes, allowed to warm to room temperature, cooled back to 0°C and quenched with water (100 mL). EtOAc (400 mL) was then added, and organic layer separated, washed with water (2x), brine (lx), dried (MgS04), filtered and concentrated in vacuo to leave a brown solid. The solid was triturated in pentane (lOOmL) for10 minutes, then filtered. The filtrate was concentrated in vacuo to afford product as a brown011 that turned to a crystalline solid on standing, 11.85 g, 89percent). XH NMR (DMSO-d6) δ 8.78 (s, 1H), 8.76 (s, 1H). [00372] To a solution of 3-bromo-4-chloro-5-fluoro-pyridine (7.56 g, 32.18 mmol) in pentane (100 mL) was added hydrogen chloride (2M in ether) (17.7 mL of 2 M, 35.4 mmol). An off-white precipitate formed instantly. The mixture was stirred for 5 minutes then the solid was collected by filtration, washed with pentane and dried by suction to afford the desired product as an off-white solid (4.79 g, 60percent). XH NMR (DMSO-d6) δ 8.77 (s, 1H), 8.75 (s, 1H). |
| 89% |
Stage #1: With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78 - -70℃; for 1 h;
Stage #2: With hexachloroethane In tetrahydrofuran; hexane at -78 - -70℃; for 0.833333 h; |
Preparation 10: (l-(3-amino-5-fluoropyridin-4-yl)piperidin-4-yl)(4-methylpiperazin-l- yl)methanone (hydrochloride) 17b Scheme 5 Step 1: 3-bromo-4-chloro-5-fluoropyridine hydrochloride 18 [00261] To a solution of diisopropylamine (6.899 g, 9.555 mL, 68.18 mmol) in THF (75 mL) cooled to -78°C, was added butyllithium (25 mL of 2.5 M in hexanes, 62.5 mmol). The reaction mixture was allowed to warm to -20°C then cooled back down to -78°C. A solution of 3-bromo-5-fluoro-pyridine (10 g, 56.82 mmol) in THF (25 mL) was added dropwise keeping temperature below -70°C (approx 30 mins). The reaction mixture was stirred at - 78°C for 30 min and a solution of 1, 1, 1,2,2,2-hexachloroethane (14.8 g, 62.5 mmol) in THF (20 mL) was then added dropwise, keeping temperature below -70°C (over approximately 30 mins). The mixture was stirred at -78°C for 20 minutes, allowed to warm to room temperature, cooled back to 0°C and quenched with water (100 rnL). EtOAc (400 mL) was then added, and organic layer separated, washed with water (2x), brine (lx), dried (MgS04), filtered and concentrated in vacuo to leave a brown solid. The solid was triturated in pentane (lOOmL) for 10 minutes, then filtered. The filtrate was concentrated in vacuo to afford product as a brown oil that turned to a crystalline solid on standing, 1 1.85 g, 89percent). lH NMR (DMSO-d6) δ 8.78 (s, 1H), 8.76 (s, 1H). [00262] To a solution of 3-bromo-4-chloro-5-fluoro-pyridine (7.56 g, 32.18 mmol) in pentane (100 mL) was added hydrogen chloride (2M in ether) (17.7 mL of 2 M, 35.4 mmol). An off-white precipitate formed instantly. The mixture was stirred for 5 minutes then the solid was collected by filtration, washed with pentane and dried by suction to afford the desired product as an off-white solid (4.79 g, 60percent). XH NMR (DMSO-d6) δ 8.77 (s, 1H), 8.75 (s, 1H). |
| 89% |
Stage #1: With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78 - -70℃; for 1 h;
Stage #2: With hexachloroethane In tetrahydrofuran; hexane at -78 - -70℃; for 0.833333 h; |
Preparation 9: (l-(3-amino-5-fluoropyridin-4-yl)piperidin-4-yl)(4-methylpiperazin-l- yl)methanone (hydrochloride) 17 Scheme 4 Step 1: 3-bromo-4-chloro-5-fluoropyridine hydrochloride 18 [00264] To a solution of diisopropylamine (6.899 g, 9.555 mL, 68.18 mmol) in THF (75 mL) cooled to -78°C, was added butyllithium (25 mL of 2.5 M in hexanes, 62.5 mmol). The reaction mixture was allowed to warm to -20°C then cooled back down to -78°C. A solution of 3-bromo-5-fluoro-pyridine (10 g, 56.82 mmol) in THF (25 mL) was added dropwise keeping temperature below -70°C (approx 30 mins). The reaction mixture was stirred at - 78°C for 30 min and a solution of 1, 1, 1,2,2,2-hexachloroethane (14.8 g, 62.5 mmol) in THF (20 mL) was then added dropwise, keeping temperature below -70°C (over approx 30 mins). The mixture was stirred at -78°C for 20 minutes, allowed to warm to room temperature, cooled back to 0°C and quenched with water (100 mL). EtOAc (400 mL) was then added, and organic layer separated, washed with water (2x), brine (lx), dried (MgS04), filtered and concentrated in vacuo to leave a brown solid. The solid was triturated in pentane (lOOmL) for 10 minutes, then filtered. The filtrate was concentrated in vacuo to afford product as a brown 011 that turned to a crystalline solid on standing, 11.85 g, 89percent). 1H NMR (DMSO-d6) δ 8.78 (s, 1H), 8.76 (s, 1H). [00265] To a solution of 3-bromo-4-chloro-5-fluoro-pyridine (7.56 g, 32.18 mmol) in pentane (100 mL) was added hydrogen chloride (2M in ether) (17.7 mL of 2 M, 35.4 mmol). An off-white precipitate formed instantly. The mixture was stirred for 5 minutes then the solid was collected by filtration, washed with pentane and dried by suction to afford the desired product as an off-white solid (4.79 g, 60percent). 1H NMR (DMSO-d6) δ 8.77 (s, 1H), 8.75 (s, 1H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping