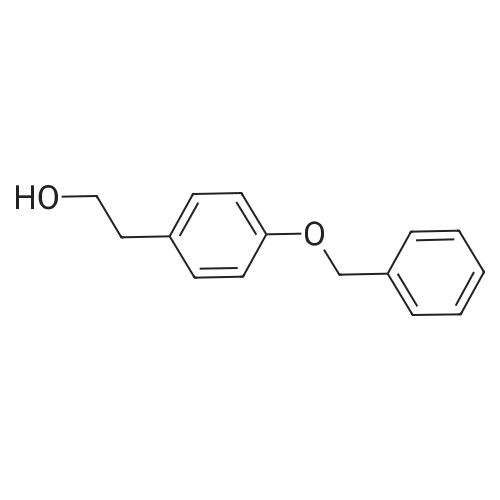
Catalytic Selective Oxidation of β-O-4 Bond in Phenethoxybenzene as a Lignin Model Using (TBA)5[PMo10V2O40] Nanocatalyst: Optimization of Operational Conditions
Diaz, Juan
;
Pizzio, Luis R.
;
Pecchi, Gina
, et al.
Molecules,2023,28(17):6368.
DOI:
10.3390/molecules28176368
PubMed ID:
37687197
More
Abstract: The catalytic oxidation of phenethoxybenzene as a lignin model compound with a β-O-4 bond was conducted using the Keggin-type polyoxometalate nanocatalyst (TBA)5[PMo10V2O40]. The optimization of the process′s operational conditions was carried out using response surface methodol. The statistically significant variables in the process were determined using a fractional factorial design. Based on this selection, a central circumscribed composite exptl. design was used to maximize the phenethoxybenzene conversion, varying temperature, reaction time, and catalyst load. The optimal conditions that maximized the phenethoxybenzene conversion were 137 °C, 3.5 h, and 200 mg of catalyst. In addition, under the optimized conditions, the Kraft lignin catalytic depolymerization was carried out to validate the effectiveness of the process. The depolymerization degree was assessed by gel permeation chromatog. from which a significant decrease in the molar mass distribution Mw from 7.34 kDa to 1.97 kDa and a reduction in the polydispersity index PDI from 6 to 3 were observed Furthermore, the successful cleavage of the β-O-4 bond in the Kraft lignin was verified by gas chromatog.-mass spectrometry anal. of the reaction products. These results offer a sustainable alternative to efficiently converting lignin into valuable products.
Keywords:
polyoxometalate nanocatalyst ;
Keggin-type ;
lignin model compound ;
beta-O-4 bond ;
heterogeneous catalysis ;
green chemistry
Purchased from AmBeed:
40515-89-7
5[PMo10V2O40] Nanocatalyst Optimization of Operational Conditions.png)
Hierarchical porous activated carbon-supported ruthenium catalysts for catalytic cleavage of lignin model compounds
Pham, Xuan-Tien
;
Tran, Vy Anh
;
Tran, Lan-Trinh Thi
, et al.
Energies,2022,?15(22):8611.
DOI:
10.3390/en15228611
More
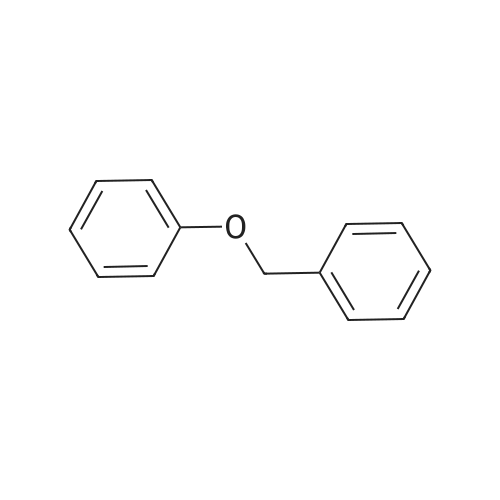
Abstract: The catalytic conversion of lignin model compounds was performed using Ru/C catalysts and an autoclave reactor. The Ru/C catalysts were prepared by the impregnation method using highly porous homemade activated carbon and characterized by XRD, SEM, and specific surface area. The catalytic reactions were performed in a high pressure/temperature reactor at different temperatures and with different solvents. The results showed that the novel Ru/C catalysts prepared from carbon supports activated by the KOH agent showed higher catalytic activity than the commercial catalyst. Ethanol and 2-propanol were suitable solvents for the cleavage of the β–O–4 ether bond of 2-phenoxy1-phenyl ethanol (~65–70% conversion) over a Ru/C-KOH-2 catalyst at 220 ?C in comparison to tert-butanol and 1-propanol solvents (~43–47% conversion of 2-phenoxy-1-phenyl ethanol). Also, the increase in reaction temperature from 200 ?C to 240 ?C enhanced the cleavage of the ether bond with an increase in phenol selectivity from 9.4% to 19.5% and improved the catalytic conversion of 2-phenoxy-1-phenyl ethanol from 46.6% to 98.5% over the Ru/C-KOH-2 catalyst and ethanol solvent. The Ru/C-KOH-2 catalyst showed outstanding conversion (98.5%) of 2-phenoxy-1-phenylethanol at 240 ?C, 1 h, ethanol solvent. This novel hierarchical porous activated carbon-supported ruthenium catalyst (Ru/C-KOH-2) can be applied for the further conversion of the lignin compound.
Keywords:
active carbon ;
biochar ;
Ru/C ;
lignin ;
β-O-4 aryl ether
Purchased from AmBeed:
4249-72-3 ;
40515-89-7

Development of catalysts for the valorization of lignin: hydrodeoxygenation of dimeric aryl ethers
Rafael, Raphaela Azevedo
;
Université de Lille,2022.
More
Abstract: The present thesis aims at studying the role of the support and metal particle size on the hydrodeoxygenation reactions (HDO) of benzyl phenyl ether (BPE), phenethoxybenzene (PEB), and diphenyl ether (DPE) chosen as model molecules representative of the main ether linkages present in the lignocellulosic biomass. The reactions were carried out in the liquid phase at 230 °C and 18 bar of H2. Pd-supported on different oxides (SiO2, TiO2, Nb2O5, Al2O3, ZrO2, and HZSM5) were synthesized by incipient wetness impregnation and deposition of metal particles prepared by the colloidal method. The acidic sites of the support promote the cracking of the C-O ether bond of BPE, but for PEB and DPE, this effect is less pronounced due to the higher energy required to break these linkages. The hydrogenolysis of the C-O ether bond takes place on the metallic Pd particles, producing the respective arenes. However, the Pd particle size can directly affect the product distribution after C-O cleavage. Due to the larger Pd particle size, impregnated catalysts favor the hydrogenolysis and exhibit a higher selectivity to alkylated products, whereas a smaller Pd particle size, obtained for catalysts prepared by the colloidal route, increases the selectivity to deoxygenated products. Over these catalysts, the formation of alkylated products is suppressed, but the hydrogenation of BPE, PEB, and DPE aromatic rings occurs in parallel to hydrogenolysis also promoted by Pd particles. The performance of Ru-based catalysts was also evaluated in the same reaction conditions. In the presence of Ru, alkylated products are produced even in the absence of acidic sites.
Keywords:
Hydrodeoxygenation ;
Hydrogenolysis ;
Alkylation ;
Ether linkage
Purchased from AmBeed:
40515-89-7


 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping


5[PMo10V2O40] Nanocatalyst Optimization of Operational Conditions.png)