|
With acetic acid; In cyclohexane; at 20℃; for 0.5h;Purification / work up; |
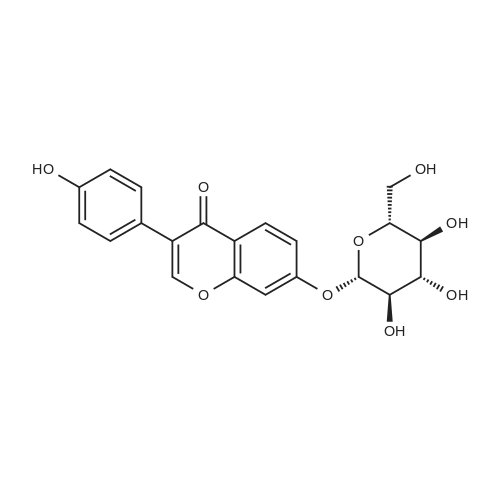
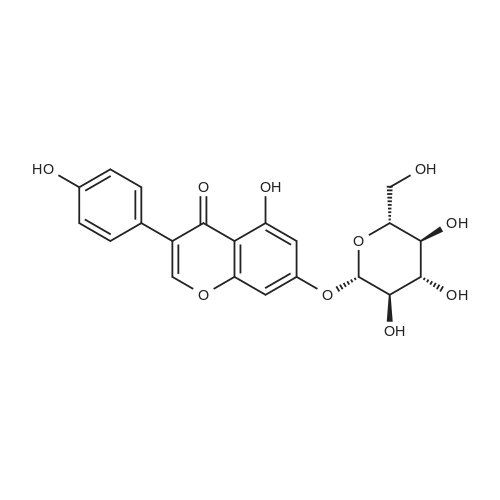
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |
|
With acetic acid; In hexane; acetone; at 20℃; for 0.5h;Purification / work up; |
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |
|
With acetic acid; In hexane; at 20℃; for 0.5h;Purification / work up; |
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |
|
With acetic acid; In water; at 20℃; for 0.5h;Purification / work up; |
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |
|
With acetic acid; In Octanoic acid; at 20℃; for 0.5h;Purification / work up; |
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |
|
With acetic acid; In acetone; at 20℃; for 0.5h;Purification / work up; |
10.0 gram aliquots of"Solgen 40" (containing 26.9% genistin, 11.9% daidzin, 2.0% glycetin; negligible aglycon content; genistin-to-daidzin ratio 2.3 : 1) were charged to a 100 ml ERLENMEYER flask equipped with a magnetic stirring bar. Varying amounts of solvents comprising glacial acetic acid (GAA) and various co-solvents were added and the resulting slurry was stirred at ambient temperature for 30 minutes, then filtered on a Buechner funnel through Whatman No.541 paper. The filter cakes were washed with 10 milliliters of the solvent employed, dried in vacuo at 80C., and subjected to HPLC analysis. The results are tabulated below. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping