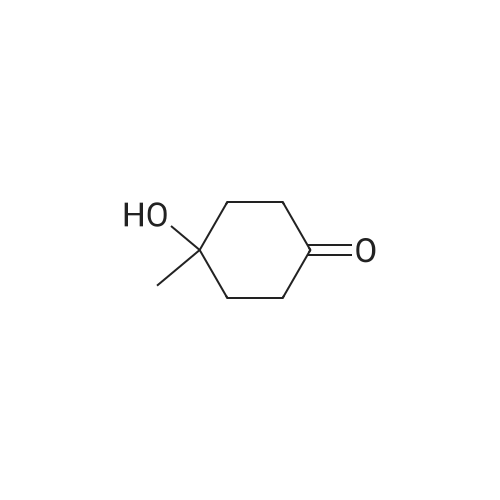
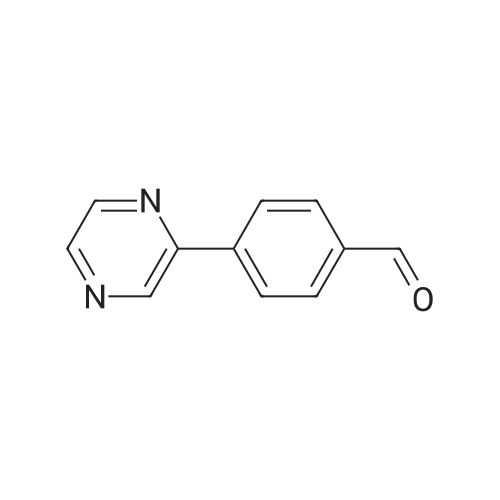
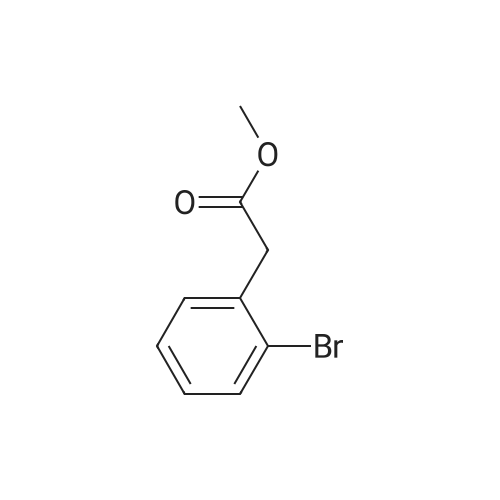
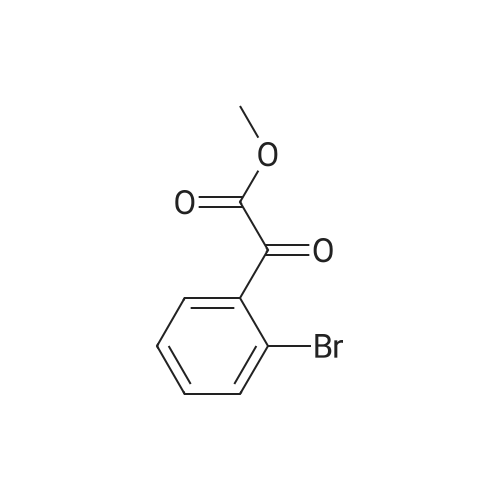
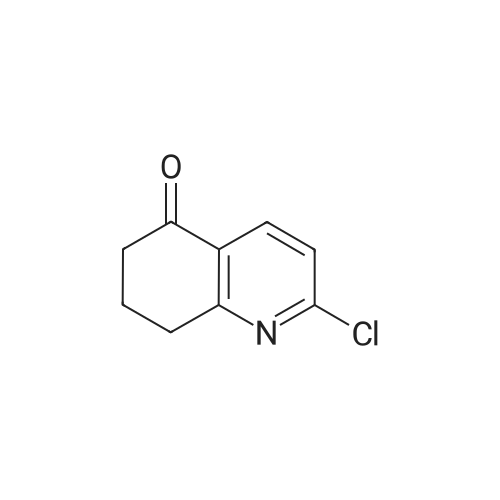
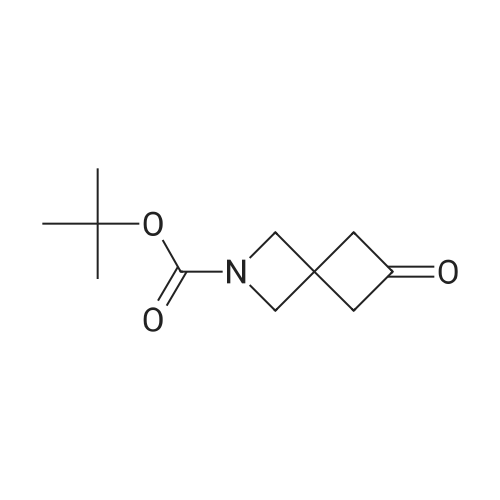
|
With sodium hydride; In tetrahydrofuran; at 0 - 20℃; for 13h;Heating / reflux; |
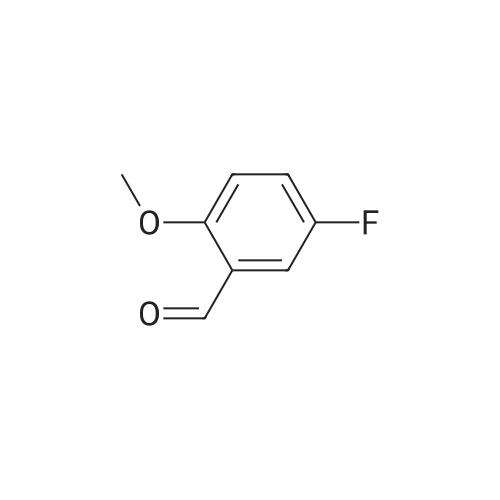
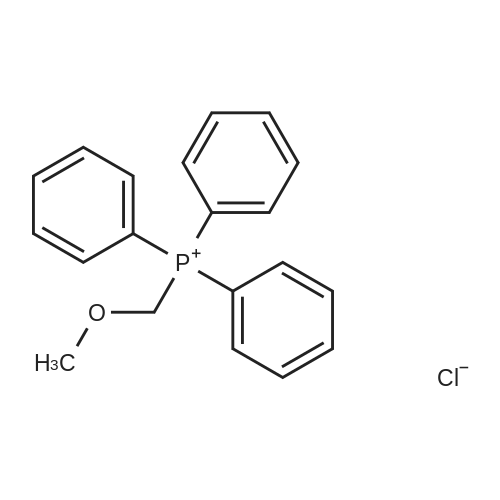
Example 3 : rralphaw.s'-2-(ammome1hyl)-5-(5'-fluoro-2'-methoxybenzyl)tetrahydrofuran[0195] S-Fluoro-2-methoxyphenacetaldehyde:; To a suspension of methoxymethyltriphenylphosphonium chloride (10.0 g, 30.0 mmol) in THF (30 mL) under Ar(g) was added NaH (60% in mineral oil, 1.2 g, 30 mmol) and the mixture thus obtained was heated to reflux for 1 h. The orange suspension thus obtained was cooled to O0C and 5- fluoro-2-methoxybenzaldehyde (4.2 g, 26.0 mmol) was added and the reaction was continued for 12 h while warmed to room temperature. The reaction mixture was poured in to a separatory funnel containing ammonium chloride aqueous solution (sat. NH4Cl: H2O=I :1, 100 mL). The organic fraction was extracted with ethyl acetate (3 x 80 mL) and the combined organic layers were washed with brine (60 mL) and dried over sodium sulfate. The solvent was then removed in vacuo and crude product thus obtained was dissolved in acetone (50 mL) and H2SO4 (1 M, 1.5 mL) was added and the mixture thus obtained was heated to reflux for 6 h. It was then cooled to room temperature and the crude product obtained after the removal of the solvent was purified by flash column chromatography (10% ethyl acetate in hexane, Rf = 0.15) to give the product (2.63 g, 66%) as an oil.; [0287] 5-Fluoro~2-methoxyphenylacetaldehyde (2a): To a suspension of methoxymethyltriphenylphosphonium chloride (10.0 g, 30.0 mmol) in THF (30 niL) under Ar was added NaH (60% in mineral oil, 1.2 g, 30 mmol) and then heated to reflux for 1 h. The orange colored suspension was cooled to 0C and <strong>[19415-51-1]5-fluoro-2-methoxybenzaldehyde</strong> (4.2 g, 26.0 mmol) was added and the mixture was stirred for 12 h at room temperature. The reaction mixture was then poured into a separatory funnel containing ammonium chloride aqueous solution (sat. NH4C1/H20, 1:1, v:v, 100 niL). The organic material was extracted with ethyl acetate (3 x 80 mL) and the combined organic layers were washed with brine (60 mL) and dried over Na2SO4. The solvent was then removed in vacuo and the crude product (2a) obtained was dissolved in acetone (50 mL). H2SO4 (1 M, 1.5 mL) was then added and the mixture obtained was heated to reflux for 6 h while stirring. The reaction was then cooled to room temperature and the solvent was removed in vacuo to obtain the crude product that was then purified by flash column chromatography (Rf = 0.15, EtOAc/Hexane, 10:90, v:v) to afford the product (2.63 g, 66%) as an oil: 1H NMR (CDCl3, 500 MHz): delta 9.68 (s, 1 H), 6.97 (dt, J= 3.1, 8.7 Hz, 1 H), 6.89 (dd, J= 3.1, 8.7 Hz, 1 H), 6.83 (dd, J= 4.3, 8.7 Hz, 1 H), 3.80 (s, 3 H), 3.63 (d, J= 1.7 Hz, 2 H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping