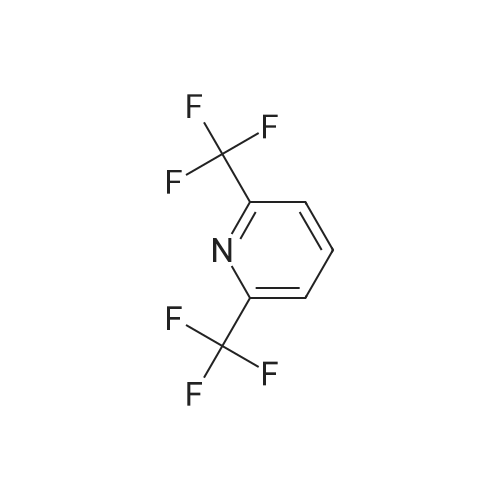
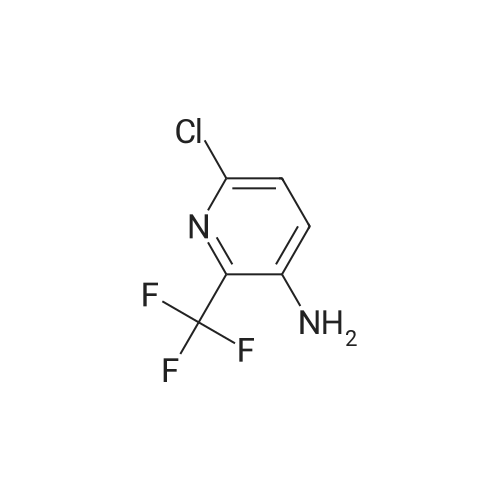
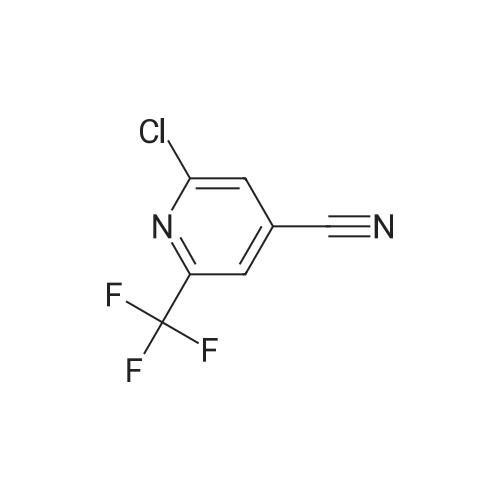
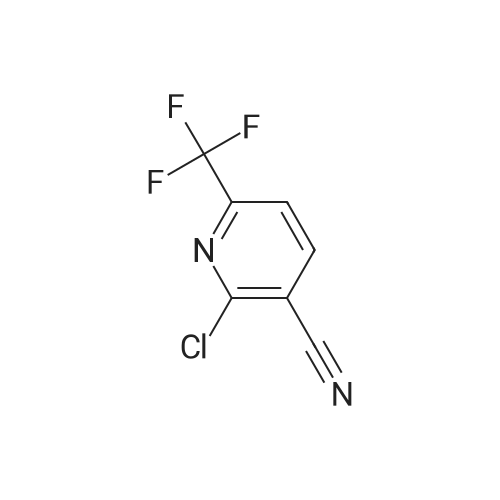
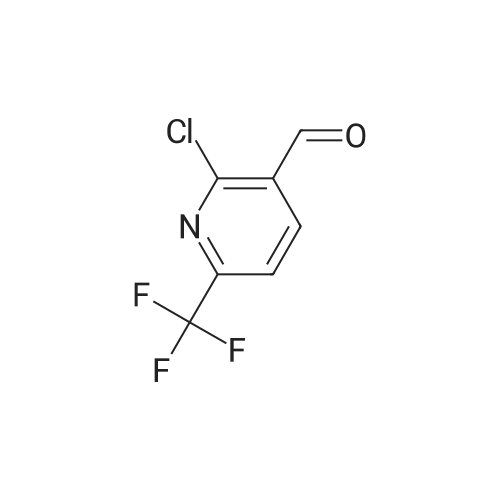
| 96% |
With triethylamine;1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; at 60℃; for 17h; |
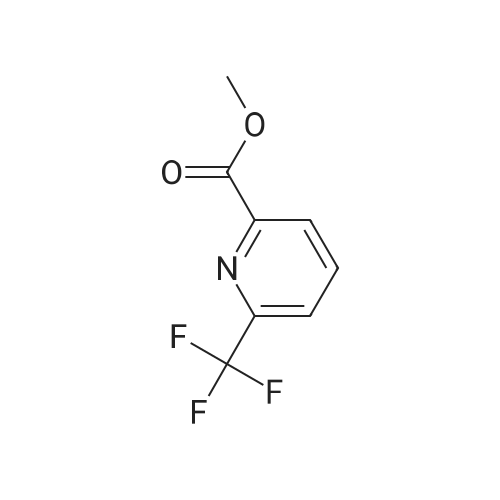
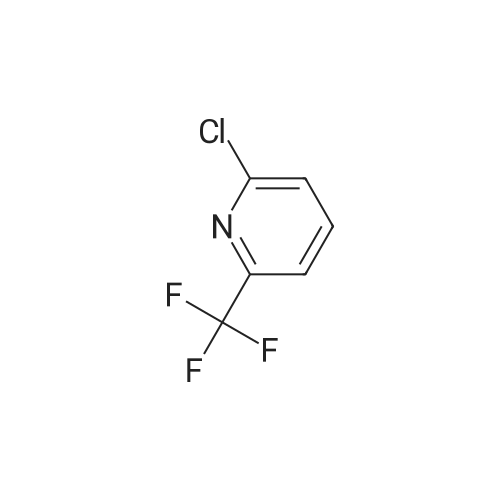
Add palladium(II) acetate (200 mg, 2 w/w%) and l,l '-bis(diphenylphosphino)ferrocene (dppf) (400 mg, 4 w/w%) to a solution of 2-chloro-6-trifluoromethyl-pyridine (10.0 g, 55.1 mmol) in methanol (30 mL). To the orange solution add triethylamine (8.45 mL, 60.6 mmol). Purge the mixture with nitrogen and then maintain under an atmosphere of carbon monoxide (40 psig) at 60 0C for 17 h. Cool to ambient temperature and concentrate under a reduced pressure. Dissolve the solid in tert-butylmethyl ether (70 mL). Filter the resulting slurry over silica gel (10 g) and diatomaceous earth (10 g).Concentrate the filtrate to afford the title compound as a light orange solid (10.8 g, 96%). 1H NMR (399.84 MHz, DMSO d6): 8.31-8.27 (m, IH), 8.14 (dd, J= 2.4, 6.4 Hz, 2H), 3.91 (s, 3H). HRMS (ESI) m/z (M+H)+ calcd for C8H7F3NO2: 206.0423, found 206.0422. |
|
With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; triethylamine; at 60℃; for 22h; |
Step 1: Preparation of 6-trifluomethyl-pyridine-2-carboxylic acid methyl ester (2) To a solution of 2-chloro-6-trifluoromethyl-pyridine (2 g, 11.1 mmol, 1.0 eq) in MeOH (20 mL) was add Pd(OAc)2 (124 mg, 0.05 eq) and dppf (600 mg, 0.1 eq) under an atmosphere of nitrogen. Et3N (2.3 mL, 1.5 eq) was then added to the resulting orange solution. The reaction solution was then stirred under an atmosphere of carbon monoxide (40 psi) at 60 C. for 22 hr. Once the reaction completed, the mixture was filtered and the filtrate was concentrated in high vacuum. The residue was purified by column chromatography to afford the desired product. 1HNMR (400 MHz, CDCl3): delta 8.32 (d, J=8 Hz, 1H), 8.06 (t, J=8 Hz, 1H), 8.88 (d, J=8 Hz, 1H), 4.04 (s, 3H). LC-MS: m/z 206 (M+H)+. |
|
With 1,1'-bis-(diphenylphosphino)ferrocene; palladium diacetate; triethylamine; In methanol; at 60℃; under 2068.65 Torr; for 22h;Inert atmosphere; |
Step 1: Preparation of 6-trfluomethyl-pyridine-2-carboxylic acid methyl ester (2). To a solution of 2-chloro-6-trifluoromethyl-pyridine (2 g, 11.1 mmol, 1.0 eq) in MeOH (20 mL) was add Pd(OAc)2 (124 mg, 0.O5eq) and dppf (600 mg, 0.leq) under an atmosphere of nitrogen. Et3N (2.3 mL, 1 .5eq) was then added to the resulting orange solution. The reaction solution was then stirred under an atmosphere of carbon monoxide (40 psi) at 60C for 22 hr. Once the reaction completed, the mixture was filtered and the filtrate was concentrated in high vacuum. The residue was purified by column chromatography to afford the desired product.?HNMR (400 MHz, CDC13): 8.32 (d, J 8 Hz, 111), 8.06 (t, J= 8 Hz, 111), 8.88 (d, J= 8 Hz, 111), 4.04 (s, 3H).LC-MS: m/z 206 (M+H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping