| 31% |
|
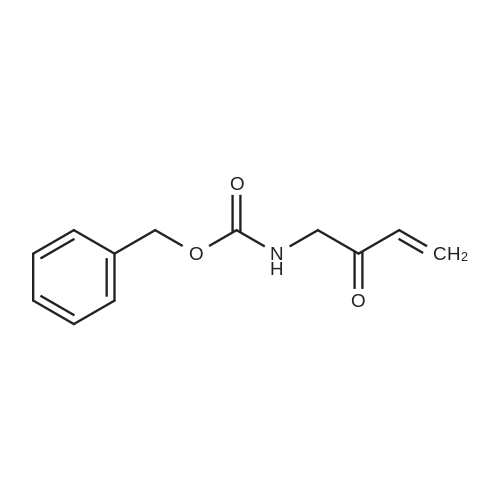
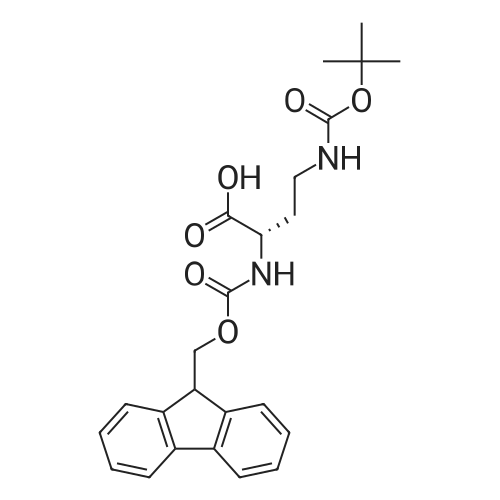
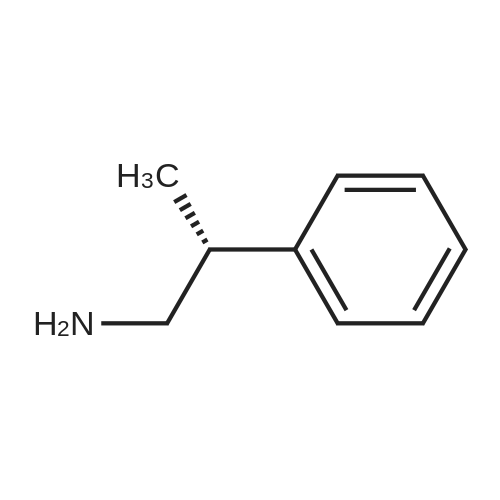
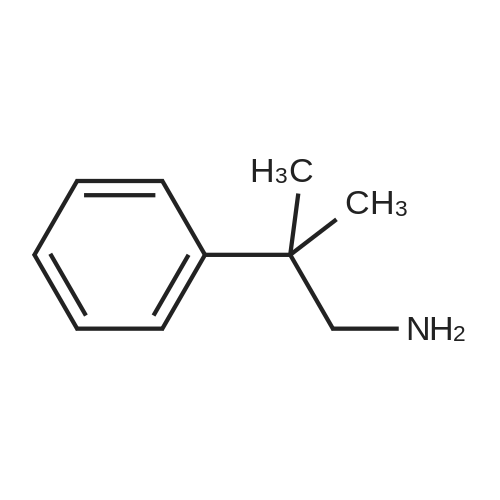
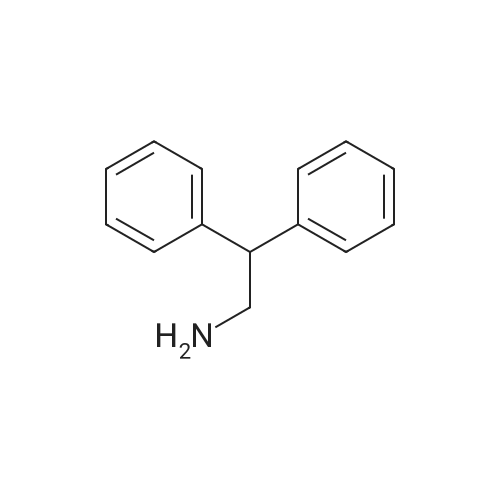
Example 17 - Synthesis of Compound 16 (S)-9-fluorenylmethyl 10-(2,2-diphenylethyl)- 2,2-dimethyl-18-phenyl-4,9,13,16-tetraoxo-3,17-dioxa-5,10,15-triazaoctadecan-8- ylcarbamate16(S)-9-fluorenylmethyl 10-(2,2-diphenylethyl)-2,2-dimethyl-18- phenyl-4,9,13,16-tetraoxo-3,17-dioxa-5,10,15-triazaoctadecan-8- ylcarbamateTo 2,2-diphenylethylamine (0.95 g, 7.4 mmol) in DCM (10 mL) was added the alphabeta- unsaturated ketone 15 (5.7 mmol) in DCM (75 mL). After stirring at room temperature for 15 mins, Fmoc-L-2,4-diaminobutyric acid(Boc)-OH (2.4 g, 8.55 mmol) and DIC (0.87 mL, 5.6 mmol) were added. The reaction was stirred at room temperature overnight. The DCM was removed by rotary evaporation and the residue was subjected to column chromatography on silica gel using petroleum spirit:EtOAc (1 :1 to 0:1 ) to give 16 (1.5 g, 31percent) Alternatively, to 2,2-diphenylethylamine (0.97 g, 7.4 mmol) in DCM (20 mL) was added the alpha,beta -unsaturated ketone 15 (5.95 mmol) in DCM (4OmL). After stirring at room temperature for 15 mins, Fmoc-L-2,4-diaminobutyric acid(Boc)-OH (2.4 g, 8.55 mmol), DIPEA (2.5 mL) and HATU (2.3 g, 6.0 mmol) were added. The reaction was stirred at room temperature overnight. The DCM was removed by rotary evaporation and the residue was taken up in EtOAc (100 mL). The organic layer was washed with saturated sodium bicarbonate solution (2x 100 mL), saturated ammonium chloride solution (2x 100 mL) and brine (2x 100 mL). The organic phase was dried and the solvent removed under reduced pressure. The residue was subjected to column chromatography on silica gel using petroleum spirit:EtOAc (3:1 to 1 :1 to 0:1 ) to give 16 (0.86 g, 17percent). |
| 31% |
|
To 2,2-diphenylethylamine (0.95 g, 7.4 mmol) in DCM (10 ml.) was added the alphabeta- unsaturated ketone 15 (5.7 mmol) in DCM (75 ml_). After stirring at room temperature for 15 mins, Fmoc-L-2,4-diaminobutyric acid(Boc)-OH (2.4 g, 8.55 mmol) and DIC (0.87 ml_, 5.6 mmol) were added. The reaction was stirred at room temperature overnight. The DCM was removed by rotary evaporation and the residue was subjected to column chromatography on silica gel using petroleum spirit:EtOAc (1 :1 to 0:1 ) to give 16 (1.5 g, 31percent) |
| 31% |
|
To 2,2-diphenylethylamine (0.95 g, 7.4 mmol) in DCM (10 mL) was added the alpha,beta-unsaturated ketone 15 (5.7 mmol) in DCM (75 mL).After stirring at room temperature for 15 mins, Fmoc-L-2,4-diaminobutyric acid(Boc)-OH (2.4 g, 8.55 mmol) and DIC (0.87 mL, 5.6 mmol) were added.The reaction was stirred at room temperature overnight.The DCM was removed by rotary evaporation and the residue was subjected to column chromatography on silica gel using petroleum spirit:EtOAc (1:1 to 0:1) to give 16 (1.5 g, 31percent)_ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping