| 55% |
With tetrafluoroboric acid; sodium nitrite; at 0℃; for 1h; |
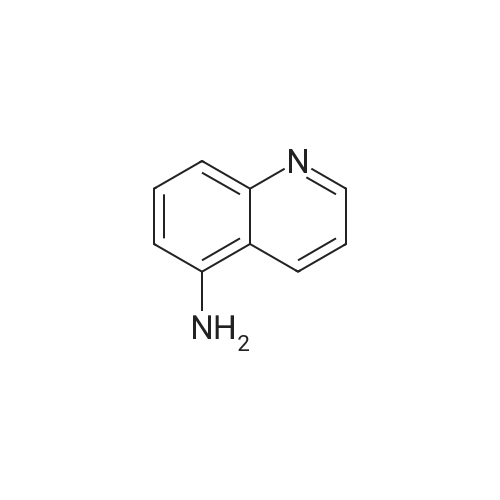
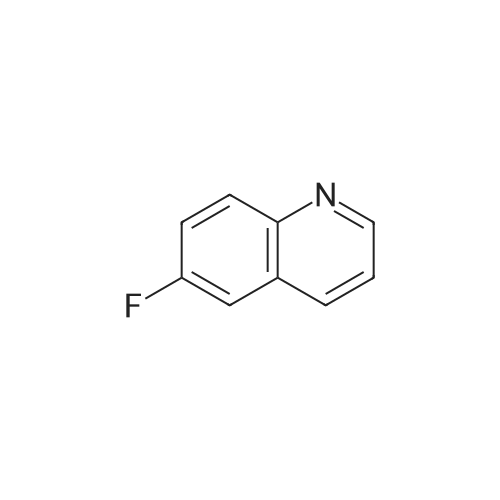
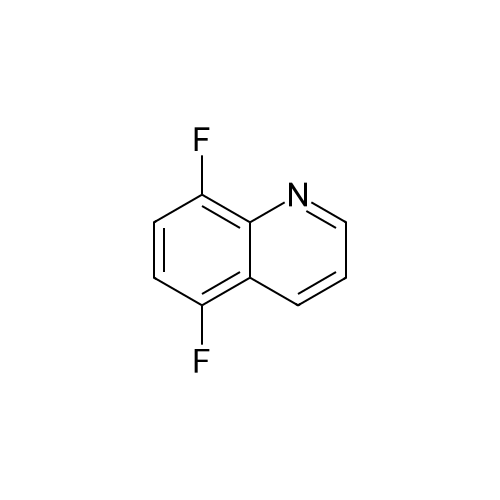
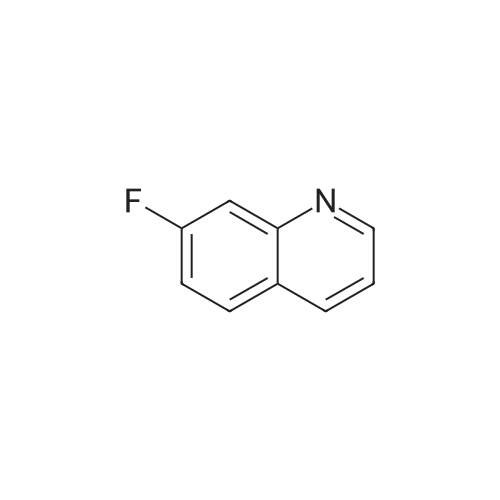
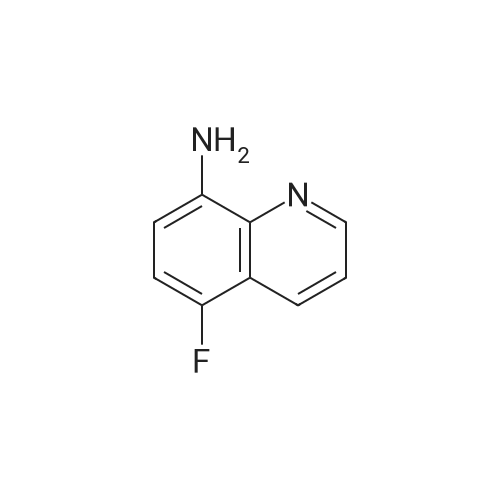
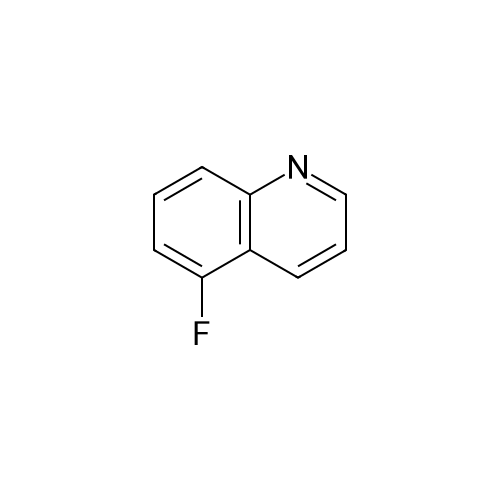
Preparation LIII 5-fluoro-1,2,3,4-tetrahydroquinoline [0363] 5-fluoroquinoline [0364] To a suspension of 5-aminoquinoline (50 g, 347 mmol) in 48percent HBF4 (200 mL) at 0° C. was added portionwise sodium nitrite. This was stirred for 1 hour and then poured into 1:1 ethyl acetate/diethyl ether (500 mL). The resulting suspension was filtered and the solid dried. This solid (82.5 g, 338 mmol) was added portionwise to refluxing xylene (1 L) and stirred for 2 hours then allowed to cool. The xylene was decanted off and the residue dissolved in 1N hydrochloric acid (600 mL). After neutralization with sodium carbonate, the mixture was extracted with ethyl acetate (10.x.500 mL). The extracts were dried over sodium sulfate, filtered and the volatiles removed under reduced pressure. The residue was subjected to silica gel chromatography, eluting with 10-20percent diethyl ether in hexanes. Fractions containing product were combined and concentrated under reduced pressure to provide 28.1 g (55percent) of the desired compound. MS (EI, m/z) C9H6FN (M+1) 148.0 [0365] Reduction [0366] A mixture of 5-fluoroquinoline (28.1 g), 5percent palladium on carbon (5.6 g) in methanol was shaken over night at 40° C. under 60 psi hydrogen. The mixture was filtered through celite and concentrated under reduced pressure. The residue was subjected to silica gel chromatography, eluting with 5-10percent ethyl acetate in hexanes. Fractions containing product were combined and concentrated under reduced pressure to provide 22.5 g (78percent) of the title compound. [0367] MS (EI, m/z) C9H10FN (M+1) 152.0 |
| 24.5% |
With tetrafluoroboric acid; sodium nitrite; In xylene; at 0℃;Reflux; |
To a suspension of 5-aminoquinoline (lO.Og, 0.069 mol) in 48percent HBF4 (40 mL) at 0°C was added portionwise sodium nitrite. This was stirred for 1 hour and then poured into 1 : 1 ethyl acetate/diethyl ether (50 mL). The resulting suspension was filtered and the solid dried. This solid was added portionwise to refluxing xylene (80 mL) and stirred for 2 hours then allowed to cool. The xylene was decanted off and the residue was dissolved in IN aqueous hydrochloric acid (100 mL). After neutralization with sodium carbonate, the mixture was extracted with ethyl acetate (3 x 80 mL). The extracts were dried over sodium sulfate, filtered and the volatiles were removed in vacuo. The residue was purified by silica gel column chromatography, eluting with 2percent ethyl acetate in petroleum ether to afford 5-fluoroquinoline as a colorless oil (2.5 g, 24.5percent).'H-NMR (300 MHz, CDC13) delta 8.93 - 8.98 (m, 1H), 8.43 - 8.46 (m, H), 7.92 (d, / = 8.4 Hz, 1H), 7.62 - 7.78 (m, 1H), 7.41 - 7.49 (m, 1H), 7.22 - 7.26 (m, 1H) |
| 800 mg |
With tetrafluoroboric acid; sodium nitrite; at 0℃; for 1h; |
To a solution of quinolin-5 -amine (2 g, 13.9 mmol) in 10 mL of 48percent HBF4 at 0°C was added sodium nitrite (933 mg, 13.5 mmol) portionwise. This was stirred for 1 hour and then poured into 1 : 1 ethyl acetate diethyl ether mixture (50 mL). The resulting suspension was filtered and the solid was dried. This solid was added portionwise to refluxing xylene (30 mL) and stirred for 3 hours, then allowed to cool. The xylene was decanted off and the residue was dissolved in IN HC1 (50 mL). After neutralization with NaHC03, the mixture was extracted with ethyl acetate (3 x 50 mL). The extracts were dried over sodium sulfate, filtered and the volatiles were removed under reduced pressure. The residue was purified by silica gel chromatography (3percent EtOAc/PE) to afford 800 mg of title compound as colorless oil. LC-MS: m/z 148.2 (M+H)+ |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping