| 92% |
With sodium tetrahydroborate In ethanol at 20℃; for 4 h; Cooling with ice |
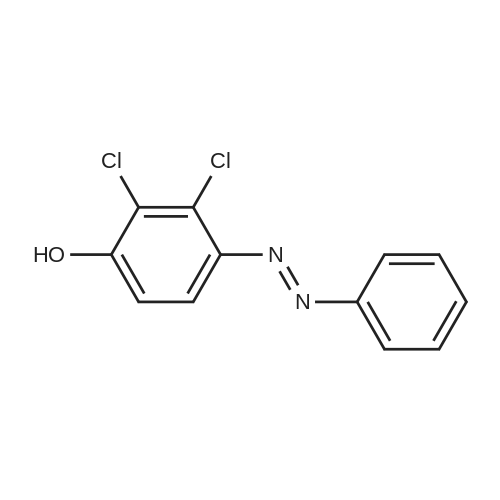
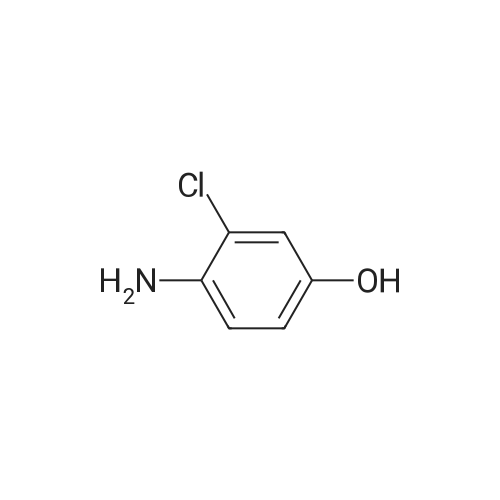
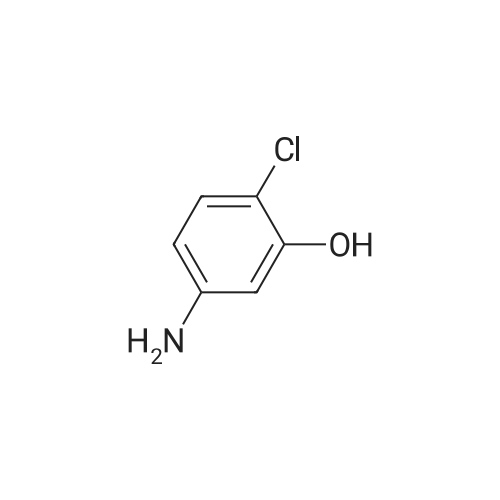
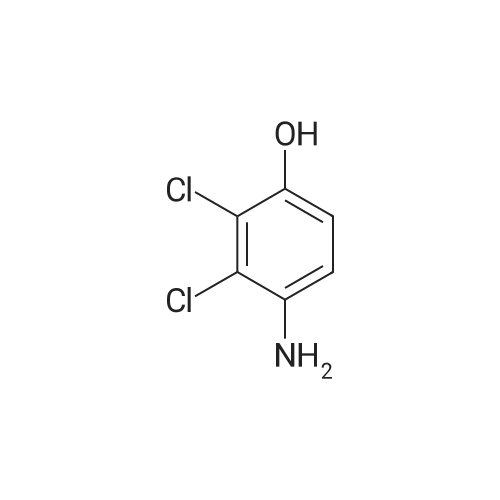
Equipped with a stirrer and a reflux condenser 100 ml two-necked flask, was added 8.0 g (0.03 mol) of starting material 2,3-dichloro-4-phenylazophenol, 1 g of sodium hydroxide dissolved in 40 ml ethanol, ice-water bath, sodium borohydride was added in small portions 4.5 g (0.12 mol). After completion of the dropwise addition, the ice bath was removed and the mixture was stirred, warmed to room temperature, the reaction was continued for 4 hours. After completion of the reaction, was added diluted hydrochloric acid solution to pH = 7, extracted three times with ethyl acetate, the combined organic phase was dried over anhydrous sodium sulfate, the solvent was distilled off rotary evaporator, was recrystallized from 20 ml of toluene, filtered and dried to give the product pale pink crystals 5.08 g, 95percent yield, 100percent pure. |
| 139 kg |
With sodium dithionite; sodium hydroxide In ethanol; water at 40℃; for 4 h; Large scale |
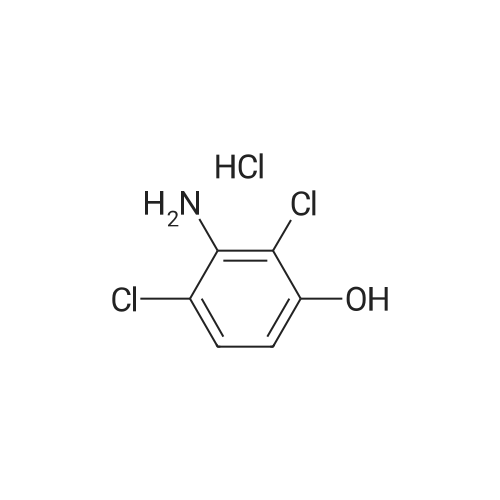
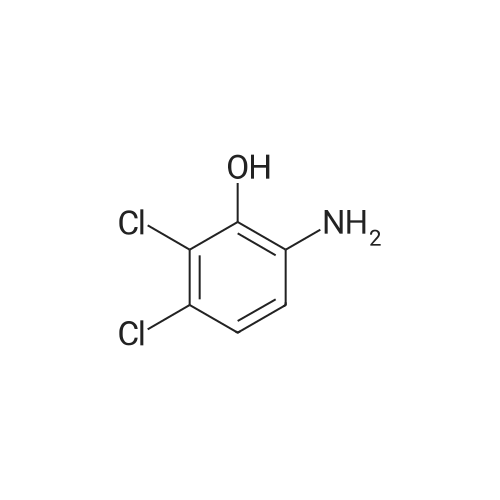
Step 3, the azo obtained in step 2 is put into a reduction kettle. 500 kg of ethanol and 500 kg of water are added, and the temperature is raised to 40 ° C. First, 12.5 kg of sodium hydroxide is added to adjust the pH to 10, and then 50 kg of insurance powder is added ( Sodium dithionite, Na2O4S2). Repeatedly add 12.5kg of sodium hydroxide and 50kg of insurance powder 7 times. After the addition, the reaction is kept at this temperature for 4 hours. The azo is reduced by the action of the powder to form crude 2,3-dichloro-4-hydroxyaniline and aniline. Subsequently, the temperature was raised to 60 ° C and ethanol, water and reduced aniline were recovered under a vacuum of -0.087 MPa, and about 700 kg of the mixture was recovered after 4 hours of recovery. After the recovery, 800 kg of water was added to the crude 2,3-dichloro-4-hydroxyaniline, the temperature was raised to 60 ° C, the temperature was kept for 1 hour, the temperature was lowered to 30 ° C, and the mixture was centrifuged at 3,300 rpm for 10 minutes to obtain 180 to 200 kg of centrifugation. The material is removed by water washing to remove impurities such as water-soluble inorganic salts; the centrifugal material is recrystallized by using 99percent toluene to remove organic impurities, specifically: the centrifuge is put into the reaction vessel, 500 kg of toluene is added, and the temperature is raised to 84 ° C. The temperature was kept at this temperature for 1 hour, the temperature was lowered to 25 ° C, and the mixture was further centrifuged at 3,300 rpm for 10 minutes, and dried at 70 ° C for 3 hours to obtain 2,3-dichloro-4-hydroxyaniline. Among them, the weight of 2,3-dichloro-4-hydroxyaniline is 138-139 kg, the content is 98.0percent, the yield is 96percent, and the specific reduction of azo to 2,3-dichloro-4-hydroxyaniline The process is as shown in equation (3). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping