| 95% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Sealed tube; Inert atmosphere; |
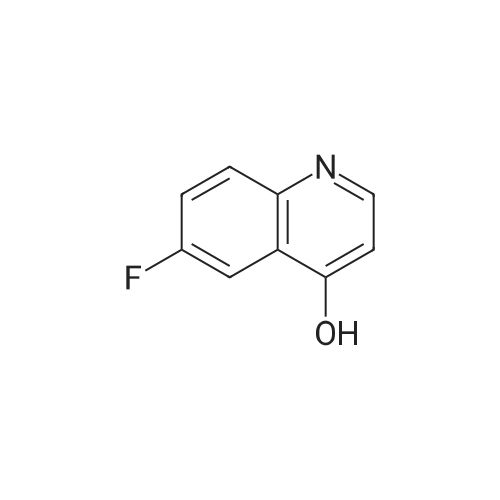
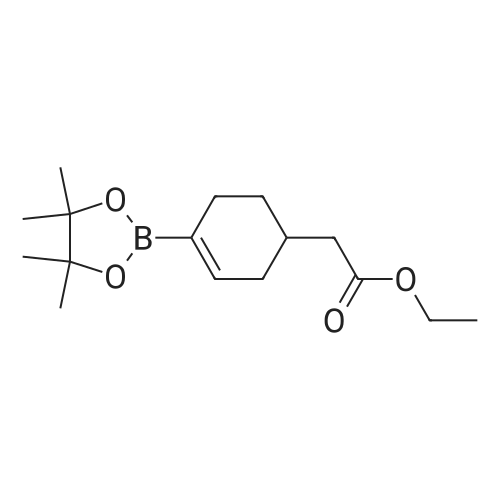
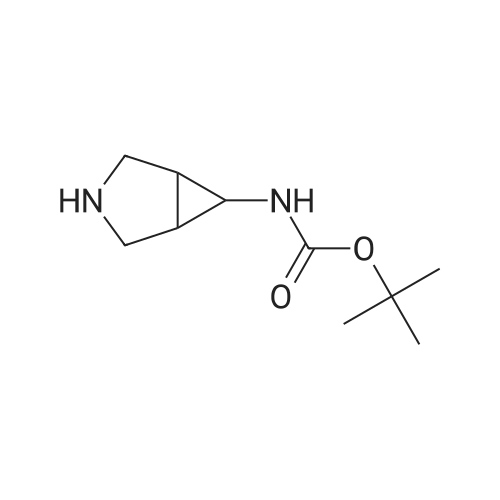
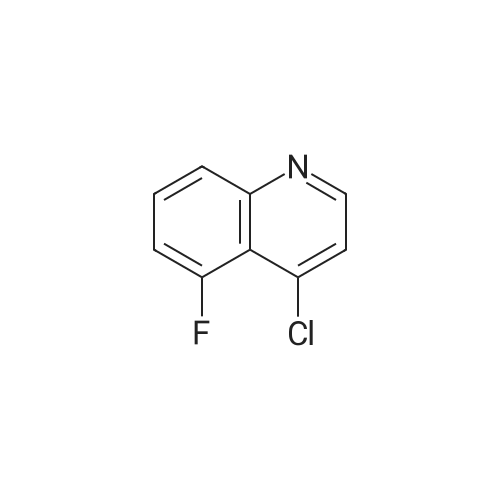
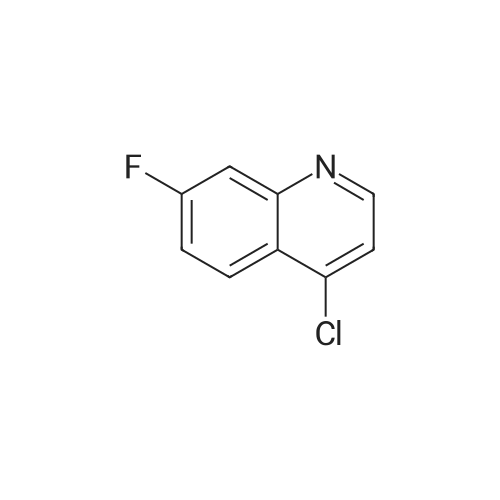
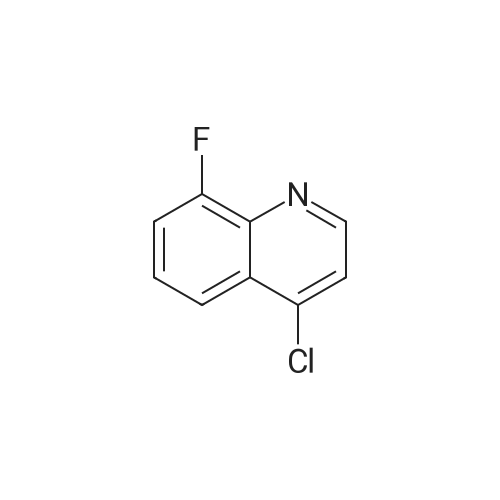
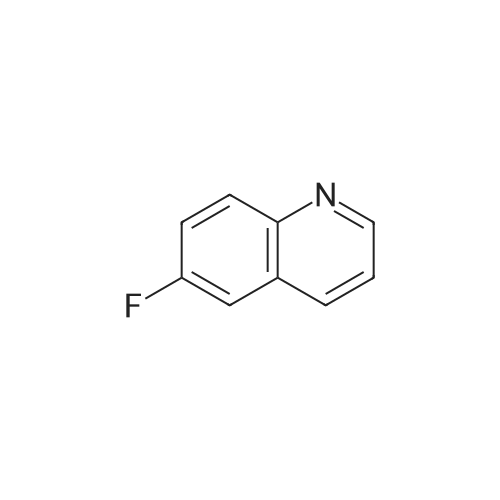
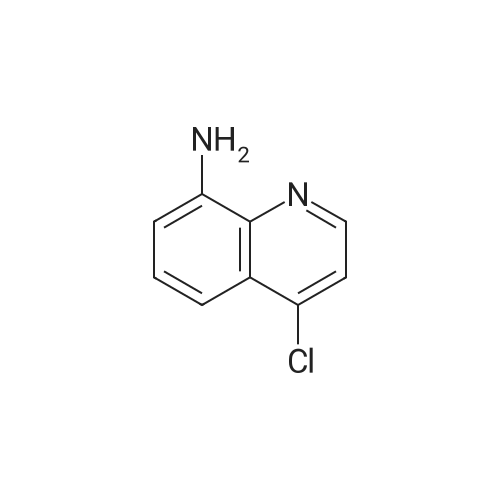
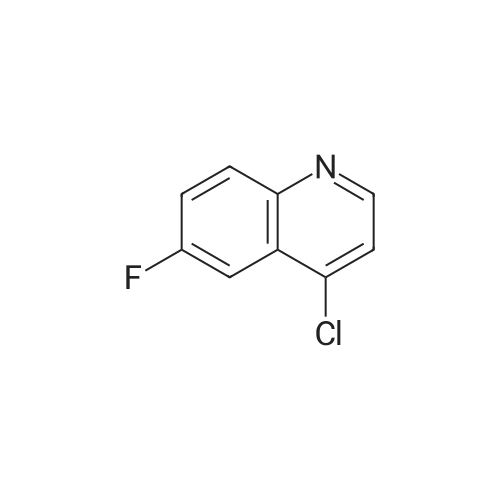
Ethyl 2-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)cyclohex-3-en-l- yl)acetate (Intermediate 154E) (5 g, 17.00 mmol) was taken up in dioxane (28.3 ml) and water (7.08 ml). 4-Chloro-6-fluoroquinoline (2.57 g, 14.15 mmol) was added followed by K2C03 (5.87 g, 42.5 mmol). Mixture was bubble with nitrogen gas for 5 minutes before the addition of Pd(Ph3P)4 (0.327 g, 0.283 mmol). After addition, reaction was vacated and backfilled with N2 three times and then sealed (sealed vial parafilmed) and heated to 100 C for 16 hours. The reaction was concentrated in vacuo and purified directly via silica gel flash column chromatography to give Intermediate 154F (4.22 g, 13.47 mmol, 95% yield). LC-MS Anal. Calc'd for C19H20FNO2 313.15, found [M+H]+ 314.1 Tr = 0.75 min (Method A |
| 95% |
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Inert atmosphere; Sealed tube; |
Ethyl 2-(4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)cyclohex-3-en-l- yl)acetate (WO 2016/073774, PCT/US 2015/059316 Intermediate 164E) (5 g, 17.00 mmol) was taken up in dioxane (28.3 ml) and water (7.08 ml). 4-chloro-6-fluoroquinoline (2.57 g, 14.15 mmol) was added followed by K2CO3 (5.87 g, 42.5 mmol). The reaction mixture was bubble with nitrogen gas for 5 minutes before the addition of Pd(Ph3P)4 (0.327 g, 0.283 mmol). After addition, reaction was vacated and backfilled with N2 three times and then sealed (sealed vial parafilmed) and heated to 100 C for 16 hours. The reaction was concentrated in vacuo and purified directly via silica gel flash column chromatography to give Intermediate 10A (4.22 g, 13.47 mmol, 95 % yield). LC-MS Anal. Calc'd for C19H20FNO2 313.15, found [M+H] 314.1 Tr = 0.75 min (Method A). |
|
With tetrakis(triphenylphosphine) palladium(0); potassium carbonate; In 1,4-dioxane; water; at 100℃; for 16h;Inert atmosphere; Sealed tube; |
Ethyl 2-(4-(4,4,5 ,5 -tetramethyl- 1,3 ,2-dioxaborolan-2-yl)cyclohex-3 -en-i - yl)acetate (Intermediate 164E) (5 g, 17.00 mmol) was taken up in dioxane (28.3 ml) and water (7.08 ml). 4-Chloro-6-fluoroquinoline (2.57 g, 14.15 mmol) was added followed by K2C03 (5.87 g, 42.5 mmol). Mixture was bubbled with nitrogen gas for 5 minutesbefore the addition of Pd(Ph3P)4 (0.327 g, 0.283 mmol). After the addition, the reaction was evacuated and backfilled with N2 three times and then sealed (sealed vial parafilmed) and heated to 100 C for 16 hours. The reaction was concentrated in vacuo and purified directly via silica gel flash colunm chromatography to give Intermediate 164F (4.22 g,13.47 mmol, 95% yield). LC-MS Anal. Calc’d for C,9H20FN02 313.15, found [M+H]314.1 T = 0.75 mm (Method A). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping