| 63% |
With diphenyl phosphoryl azide; N-ethyl-N,N-diisopropylamine; at 82℃;Inert atmosphere; |
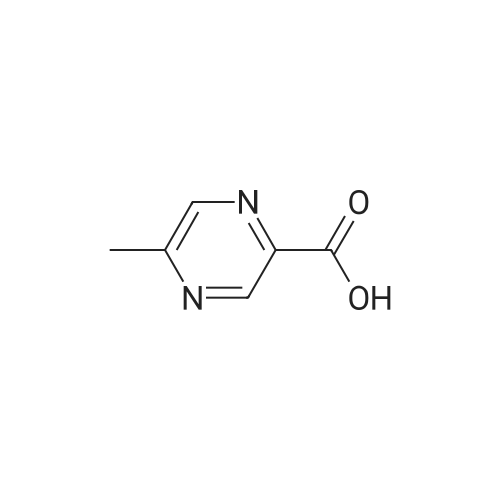
To a flask fitted with overhead stirrer, condenser, thermometer and nitrogen line was added 5-methylpyrazine-2-carboxylic acid (1.0 eq), tert-butanol (3.5 vols) and di-isopropylethylamine (1.5 eq) under a nitrogen atmosphere. The mixture was heated to 82 C., then diphenylphosphorylazide (1.0 eq) was added over a time period of 5-14 hours, maintaining the temperature of the reaction mixture at approximately 82 C. The reaction mixture was stirred for at least 1.5 hours, and then cooled to approximately 60 C. A solution of 4% w/w sodium hydroxide (1.75 eq) was added over a period of 2 hours. The mixture was cooled to 15 C. over at least 5 hours then held at 15 C. for 3 hours. The batch was then filtered, and the solid slurry washed with water (2 vols). The batch was again slurry washed with water (2 vols). After drying at 55-60 C. overnight, the desired product was obtained as a solid (corrected yield 56-63%). 1H NMR delta (400 MHz CDCl3): 9.18 (s, 1H), 8.17 (bs, 1H), 8.11 (s, 1H), 2.51 (s, 3H), 1.56 (s, 9H) |
|
With diphenyl phosphoryl azide; In 1,4-dioxane; for 12.0h;Reflux; |
Intermediate: te/t-butyl 5-methylpyrazin-2-ylcarbamate (29a) (29a)To a stirred solution of 5-methyl-2-carboxylic acid (138 g, 1.0 mol) in dioxane (1 L) was added te/t-BuOH (100 mL) and diphenylphosphoryazide (330 g) and the reaction mixture was heated at reflux for 12 hours. The reaction mixture was concentrated to dryness and the residue was purified by flash column chromatography (ethyl acetate/hexanes), then recrystalized from ether to provide te/t-butyl 5-methylpyrazin-2- ylcarbamate (29a). |
|
With diphenyl phosphoryl azide; triethylamine; In 1,4-dioxane; at 100℃; for 3.0h; |
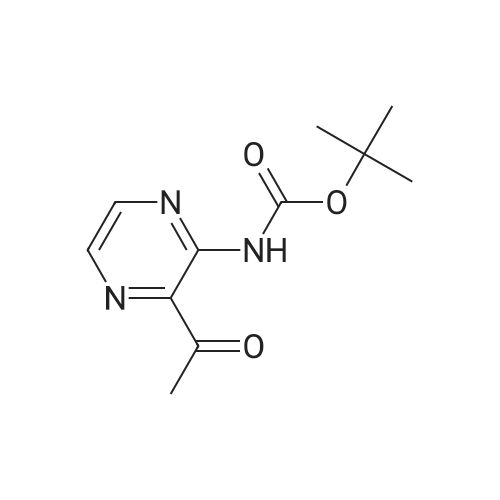
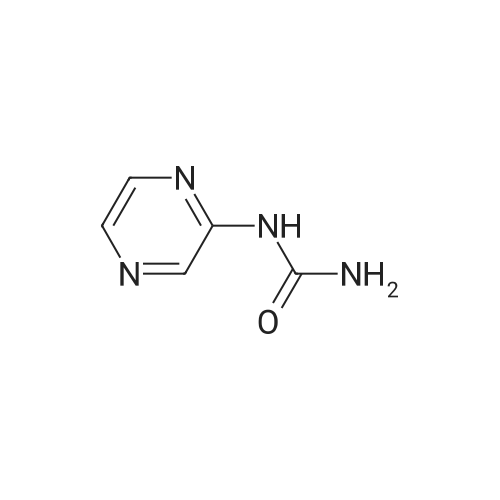
(1) 175 g of 5-methyl-2-pyrazinecarboxylic acid was suspended in 1 L of dioxane, then 1 L of t-butanol, 175 mL of triethylamine, and 287 mL of diphenyl azidophosphate were sequentially added to the suspension, and the reaction solution was heated to 100C. The obtained reaction solution was stirred at the same temperature for 3 hours and then cooled to room temperature, and the solvent was distilled off under reduced pressure. The residue was poured onto a saturated aqueous solution of sodium hydrogen carbonate, and the aqueous phase was extracted with ethyl acetate. The obtained extraction liquid was washed with a saturated aqueous solution of ammonium chloride and dried, and then the solvent was distilled off under reduced pressure. The obtained crude product was crystallized from acetonitrile, to obtain 158 g of the following Compound [1-1]. [Show Image] The spectral data of the compound represented by the above Formula [1-1] is presented below. 1H-NMR (CDCl3) delta: 9.15 (1H, s), 8.70 (1H, s), 7.41 (1H, brs), 2.51 (3H, s), 1.55 (9H, s). mass: 210 (M+1)+. |
|
With diphenyl phosphoryl azide; triethylamine; In 1,4-dioxane; at 95℃; for 3.0h; |
Step 1. tert-butyl 5-methylpyrazin-2-ylcarbamate To solution 5-methylpyrazine-2-carboxylic acid (2.5 g, 18.1 mmol), tert-butanol (6.92 mL, 72.4 mmol), Et3N (3.78 mL, 27.1 mmol) in 1,4-dioxane (12.5 mL) at 95 C. was dropwise added diphenylphosphoryl azide (DPPA, 3.23 mL, 18.1 mmol), and the reaction was heated at 95 C. for 1.5 h, followed by a 2nd portion of DPPA (1 mL, 5.6 mmol) and heated for additional 1.5 h. The reaction mixture was cooled down, concentrated and the residue was diluted with EtOAc (50 mL), washed with water (30 mL), 3 M NaOH (30 mL), sat. NaHCO3 (30 mL) and brine (30 mL), dried over Na2SO4, and concentrated. The residue was purified by flashed chromatography on silica gel eluting with gradient EtOAc/CH2Cl2 (0-20%) to afford tert-butyl 5-methylpyrazin-2-ylcarbamate as white solid. LCMS (m/z): 210.1 (MH+), 0.69 min; 1H NMR (400 MHz, CDCl3) delta ppm 9.17 (s, 1H), 8.09 (s, 1H), 7.75 (br. s., 1H), 2.51 (s, 3H), 1.56 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping