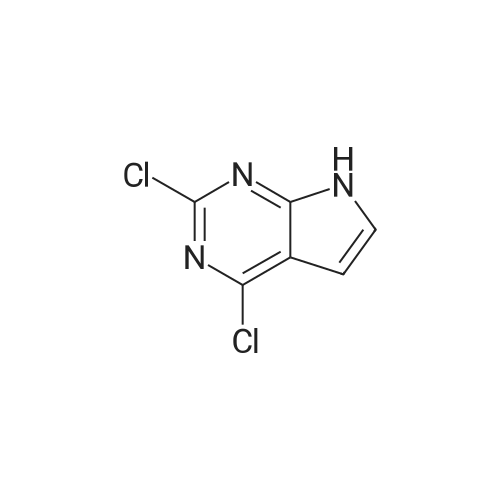
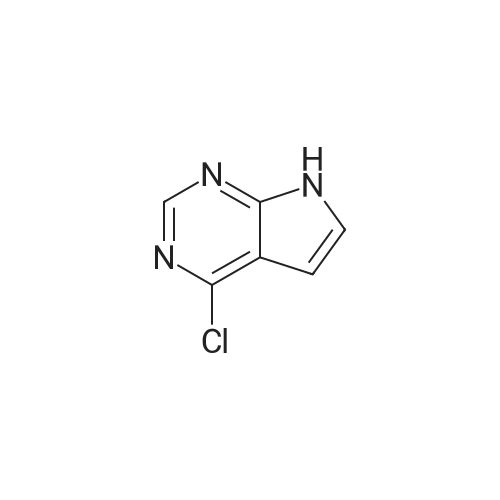
| 84.2% |
|
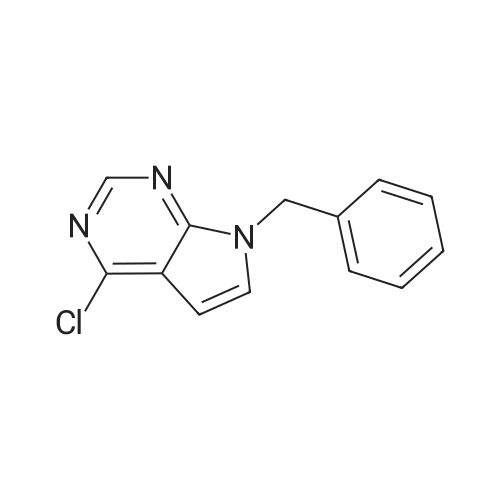
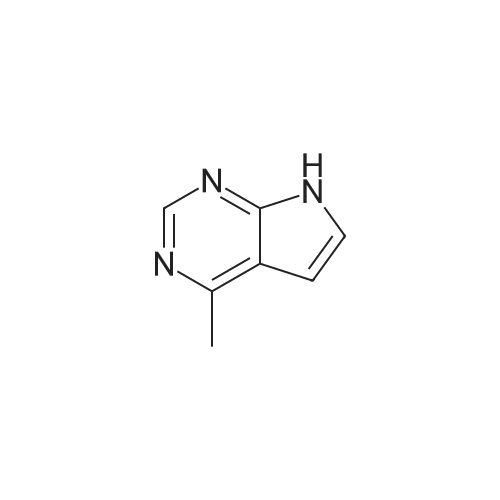
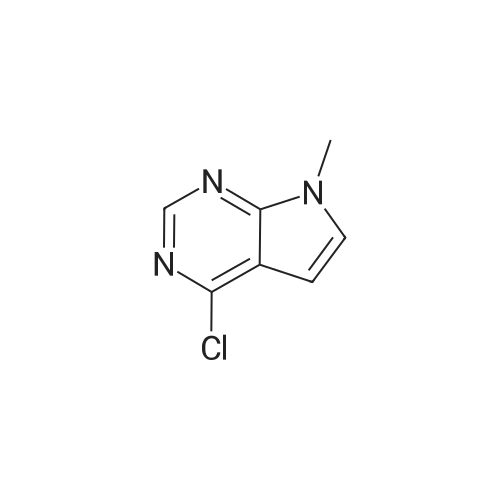
Step 1: 4-methyl-7H-pyrrolo[2,3-d]pyrimidine 150 g of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine and 5.72 g of Pd(bppf)Cl2 were added to 1.5 L of tetrahydrofuran, stirred at room temperature for 0.5 hour, and then cooled down to below 0 C. To the resulting mixture was slowly added dropwise 850 mL of methylmagnesium bromide (3M dissolved in ether). After the addition was completed, the temperature was raised to 6065 C., and the resulting mixture reacted under reflux for 2 hours. Then, the temperature was lowered to below 0 C., and the reaction was quenched by slowly adding dropwise concentrated hydrochloric acid. After the addition was completed, 650 mL of purified water was added to the resulting mixture and stirred for 15 minutes, the phases were separated and the organic phase was discarded. The aqueous phase was adjusted to pH 6 with NaHCO3 and then suction-filtered, the filter cake was washed with 455 mL of purified water, and the filtrate was collected and extracted three times with 1.05 L of ethyl acetate, and then the organic phase was concentrated to obtain 4-methyl-7H-pyrrolo[2,3-d]pyrimidine (109.5 g, 84.2%). 1H-NMR (500 MHz, DMSO-d6): delta=12.00 (bs, 1H), 8.61 (s, 1H), 7.47 (d, J=3.6 Hz, 1H), 6.62 (d, J=3.5 Hz, 1H), 2.64 (d, J=1.6 Hz, 3H); MS (ES): 134.07 (M+H+). |
|
(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; In diethyl ether; toluene; at 20 - 60℃;Inert atmosphere; |
Into a round bottom flask the catalyst PdCl2(dppf), under an atmosphere of nitrogen, was placed with 15 mL of toluene along with a stir bar. A suspension of 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (1, 1.47 g, 9.57 mmol) in 15 mL of toluene was added at room temperature. After stirring for 10 minutes, methylmagnesium bromide (17.00 mL, 3.00 M in ether, 51.00 mmol) was added dropwise. The solution turned from orange to yellow, and was slowly heated to 60 C. and stirred for 3 hrs at 60 C. and then overnight at room temperature. The resulting dark orange reaction mixture was quenched with 1 N hydrochloric acid and adjusted to pH~5, then extracted with ethyl acetate and water saturated with sodium chloride. The organic layer was washed with water and brine, dried over magnesium sulfate, filtered and the filtrate concentrated under vacuum. The resulting material was purified by silica gel column chromatography eluding with ethyl acetate and hexane. Appropriate fractions were combined and concentrated under vacuum to provide the desired compound as a yellow solid (42, 202 mg). 1H-NMR(dmso-d6) was consistent with the desired compound. MS(ESI) [M+H+]+=134.3. |
|
|
Step 1a-Preparation of 4-methyl-7H-pyrrolo[2,3-d]pyrimidine (15)To 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (9, 5.03 g, 32.8 mmol) in 100 mL of toluene, [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) 1:1 complex with dichloromethane (0.627 g, 0.328 mmol) is added under an atmosphere of nitrogen. After stirring for 10 minutes, methylmagnesium bromide (62.9 mL, 3.00 M in ether, 189 mmol) is added slowly. The reaction is heated at 55 C. overnight, then cooled to -70 to -80 C. and quenched by adding ammonium chloride dropwise. Then 1N hydrochloric acid is added and the pH is adjusted to 7-8 with the addition of saturated sodium bicarbonate. This is extracted 3× with ethyl acetate. The combined organic layer is washed with saturated ammonium chloride and brine, then dried with magnesium sulfate, filtered and the filtrate concentrated under vacuum. The resulting material is purified by silica gel column chromatography, eluting with ethyl acetate and dichloromethane, then methanol and dichloromethane. Appropriate fractions are combined and concentrated under vacuum to provide the desired compound as a tan solid (15). MS (ESI) [M+H+]+=134. This is reacted similarly to steps 2 and 3 of Scheme 4 to provide the desired compound 16. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping