| 71% |
|
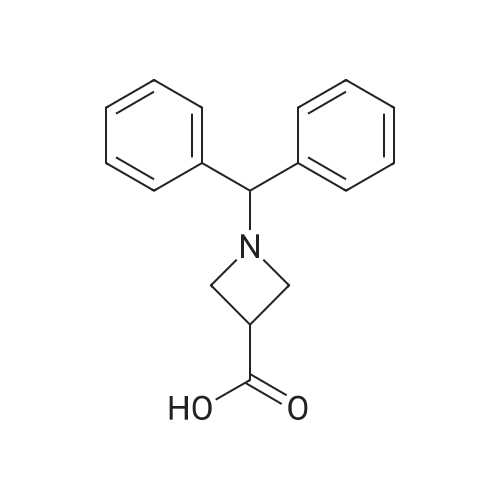
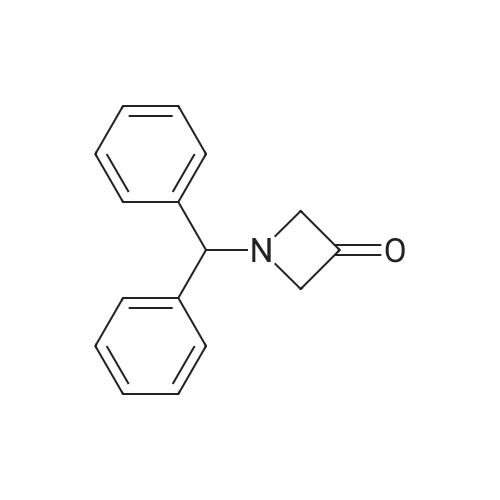
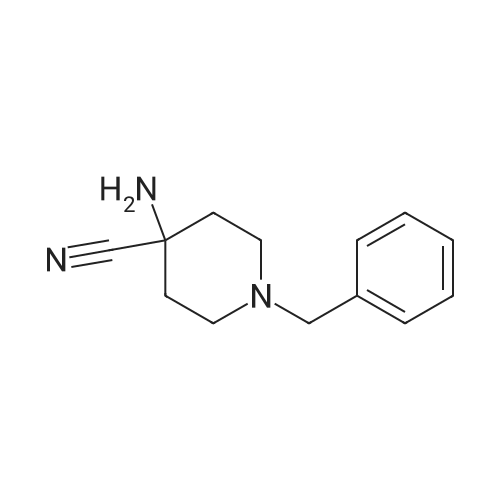
A suspension ofl-benzhydryl-azetidine-3-carbonitrile (2.09 g, 8.42 mmol) in concentrated hydrochloric acid (12 M, 15 mL) was heated at reflux for 30 minutes. The resulting solution was cooled to0 C, and 6 M sodium hydroxide was added until the mixture reached a pH of about 7. The aqueous mixture was <Desc/Clms Page number 140>then extracted with dichloromethane (3 x 150 mL) and dichloromethane: methanol (10: 1,3 x 150 mL). The combined organic layers were dried, filtered, and concentrated under reduced pressure to give the title compound (1.60 g, 71 % yield). MS (APCI): m/z 268(M+I+. |
| 71.7% |
|
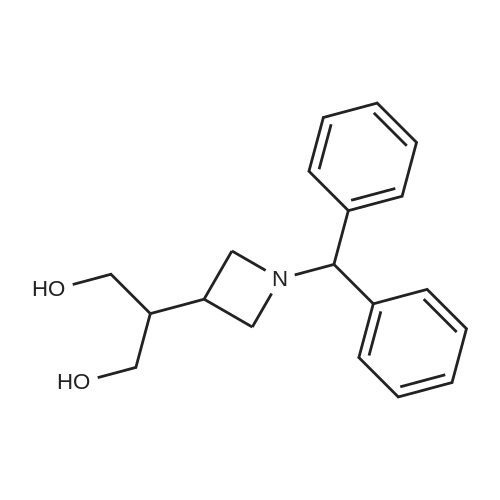
(Production Example 83) 1-Benzhydrylazetidine-3-carboxylic acid To a solution of 1-benzhydryl-3-cyanoazetidine (5.43 g) in methoxyethanol (54 ml) were added potassium hydroxide (6.48 g) and water (3.25 ml), followed by stirring at 100 C for 4 hr. The reaction mixture was allowed to cool down to room temperature. The reaction mixture was poured into ice. After adjusting this to pH 5 with 1N hydrochloric acid, sodium chloride was added thereto. This was extracted with a mixed solvent of ethyl acetate and tetrahydrofuran. The organic layer was washed with brine, and dried over anhydrous sodium sulfate. The organic layer after drying was concentrated under reduced pressure to provide a crude product of the titled compound as pale yellow crystals. The crystals were suspended by addition of diethyl ether (15 ml). The crystals were collected by filtration and washed with diethyl ether. This was dried under aeration to provide the titled compound as pale yellow crystals (4.20 g, 71.7 %). 1H-NMR Spectrum (CDCl3) δ (ppm): 3.00-3.90 (5H, m), 4.95 (1H, s), 7.25-7.28 (2H, m), 7.33 (4H, m), 7.53 (4H, m). |
|
With potassium hydroxide; In 2-ethoxy-ethanol; water; ethyl acetate; |
(1-Benzhydrylazetidin-3-yl)carboxylic acid may be prepared by carrying out the procedure in the following manner: a solution of 11 g of potassium hydroxide in 9 cm3 of water is added dropwise to a suspension, cooled to +5 C., of 14 g of (1-benzhydrylazetidin-3-yl)carbonitrile in 140 cm3 of 2-ethoxyethanol and then the mixture is heated to 95 C. After stirring for 16 hours at this temperature, the reaction mixture is poured slowly, with stirring, over ice and left at 0 C. for 68 hours and then concentrated to dryness to dryness at 50 C. under reduced pressure (2.7 kPa). The residue is taken up in 400 cm3 of water, the solution is acidified to pH 4 with 6 N hydrochloric acid and then supplemented with 400 cm3 of ethyl acetate. The resulting suspension is filtered, the solid is drained and then dried at 50 C. under reduced pressure (2.7 kPa). 13.55 g of (1-benzhydrylazetidin-3-yl)carboxylic acid are obtained in the form of a cream-colored solid. |
|
|
(3) A mixture of the above product (5.79 g), sodium hydroxide (2.57 g) and 50% aqueous ethanol (50 ml) was heated to reflux for 6 hours with stirring. After cooled to 0 C., the mixture was adjusted to pH3 with concentrated hydrochloric acid, and extracted with chloroform. The extract was sequentially washed with water and brine, and then dried over anhydrous magnesium sulfate. The solvent was removed in vacuo to give 1-diphenylmethylazetidine-3-carboxylic acid (about 6.5 g) as a yellow amorphous solid. The solid was used in the next step without any purification. |
|
In water;Acidic conditions; |
A solution of diphenylmethanamine (benzhydrylamine) and 2-oxopropane- 1,3 -diyl dimethanesulfonate were reacted to give 1 -benzhydrylazetidin-3-one 10 (Scheme 2). Reduction of the ketone of 10 with a hydride reducing agent gave 1-benzhydrylazetidin-3-ol11. Alternatively, 11 can be prepared by cyclization of benzhydrylamine and epichlorohydrin in diisopropylethylamine and ethanol. Mesylation of with methanesulfonyl chloride gave 1 -benzhydrylazetidin-3 -yl methanesulfonate 12. Displacement of the mesyl group with cyanide ion gave 1 -benzhydrylazetidine-3-carbonitrile 13. Hydrolysis of 13 with aqueous acid gave 1 -benzhydrylazetidine-3-carboxylic acid 14. Deprotection of 14 by hydrogenolysisgave 1 (CAS Reg. No.: 36476-78-5 zwitterion; 102624-46-4 hydrochloride salt; 106887-11-0 sodium salt; 1282041 potassium salt). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping