| 42% |
|
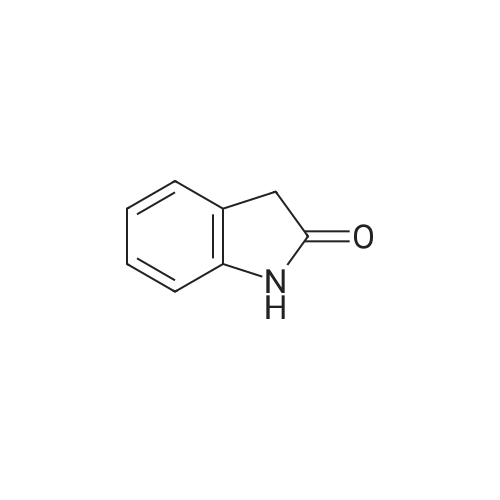
Preparation of tert-butyl 4-(2-oxoindolin- 1 -yl)piperidine- 1 -carboxylate (Compound 6-2) [00232j A mixture of Compound 6-1 (3.30 g, 20 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (4.38 g, 22 mmol), acetic acid (HOAc, 600 mg, 10 mmol) in dichloromethane (DCM, 80 mL) was stirred at room temperature for 2 h, then sodium triacetoxyborohydride (6.36 g, 30 mmol) was added in portions and heated to 40C, stirred overnight. The reaction mixture was cooled to room temperature and diluted with dichloromethane (200 mL), washed with water (200 mL x 2) and sodium bicarbonate (sat. aq., 200 mL x 2), dried over anhydrous sodium sulfate, concentrated to give the crude product, purified by column chromatography on silica gel (ethyl acetate in petroleum ether, 5%, V/v) to give Compound 6-2 (2.65 g, yield: 42%) as a white solid. MS (ES): mlz: 261[M+H-56]. ?H NMR (400 MHz, CDC13) oe: 7.24 (m, 2H), 7.01 (m, 2H), 4.41 (m, 1H), 4.28 (br s, 2H), 3.53 (s, 2H), 2.83 (br s, 2H), 2.32 (m, 2H), 1.70 (m, 2H), 1.50 (s, 9H). |
| 42% |
|
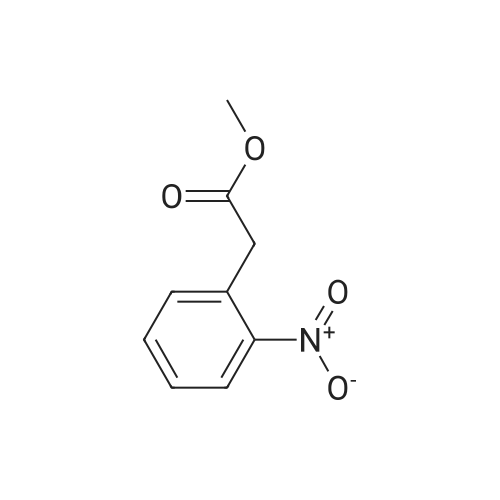
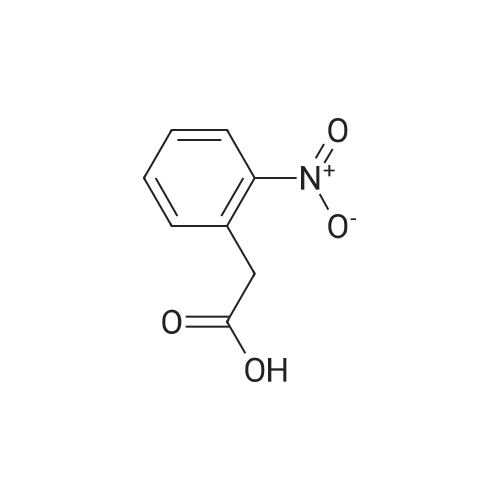
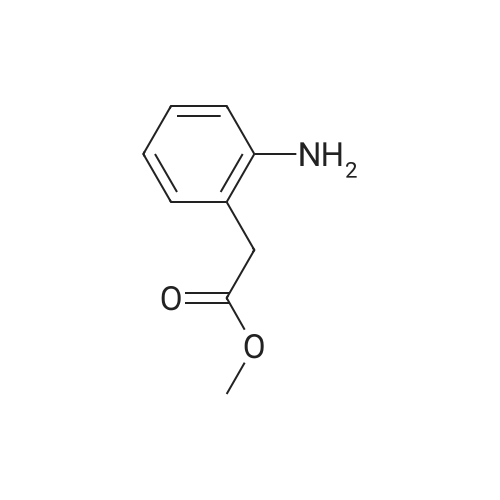
Preparation of tert-butyl 4-(2-oxoindolin- 1 -yl)piperidine- 1 -carboxylate (Compound 11-4) [00270j A mixture of <strong>[35613-44-6]methyl 2-(2-aminophenyl)acetate</strong> (Compound 11-3, 3.30 g, 20 mmol), tert-butyl 4-oxopiperidine-1-carboxylate (4.38 g, 22 mmol), acetic acid (HOAc, 600 mg, 10 mmol) in dichloromethane (DCM, 80 mL) was stirred at room temperature for 2 h, then sodium triacetoxyborohydride (NaBH(OAc)3, 6.36 g, 30 mmol) was added in portions and heated to 40C, stirred overnight. The reaction mixture was cooled to room temperature and diluted with dichloromethane (DCM, 200 mL), washed with water (200 mL x 2) and sodium bicarbonate (sat. aq., 200 mL x 2), dried over anhydrous sodium sulfate, concentrated to give the crude product, purified by flash colunm chromatography on silica gel (ethyl acetate petroleum ether, 1 20-1 15-1 10 v v) to give the desired product tert-butyl 4-(2-oxoindolin- 1 -yl)piperidine- 1- carboxylate (Compound 11-4, 2.65 g, yield: 42%) as a pale yellow solid. MS (ESI):mlz: 261[M+H-56]. ?H NMR (400 MHz, CDC13) oe: 7.24 (m, 2H), 7.01 (m, 2H), 4.41 (m, 1H), 4.28 (br s, 2H), 3.53 (s, 2H), 2.83 (br s, 2H), 2.32 (m, 2H), 1.70 (m, 2H), 1.50 (s, 9H). |
| 3.9 g (62%) |
With sodium tris(acetoxy)borohydride; sodium hydrogencarbonate; acetic acid; In dichloromethane; |
C. 4-(2-Oxo-2.3-dihydro-indol-1-yl)-piperidine-1-carboxylic acid tert-butyl ester. Methyl 2-aminophenylacetate (3.0 g, 18.2 mmol) and 1-tert-butoxycarbonyl-4-piperidone (4.5 g, 22.6 mmol) were set stirring in 50 mL of CH2Cl2 under an atmosphere of nitrogen. Sodium triacetoxyborohydride (5.4 g, 25.5 mmol) was added followed by 1 mL of acetic acid. After 20 h at rt the mixture was quenched by the slow addition of saturated NaHCO3. After stirring for 30 min, the organics were separated, dried (MgSO4), and evaporated to afford 7.5 g of a purple oil. Purification by column chromatography (silica, 10-50% EtOAc/hexanes) gave 3.9 g (62%) of the title compound. TLC (silica, 25% EtOAc/hexanes): Rf=0.15. 1H NMR (400MHz, CDCl3): 7.25 (m, 2H), 7.01 (m, 2H), 4.40 (m, 1H), 3.53 (s, 2H), 2.83 (m, 2H), 2.32 (m, 2H), 1.70 (m, 2H), 1.51 (s, 9H). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping