Alternatived Products of [ 355818-98-3 ]
Product Details of [ 355818-98-3 ]
| CAS No. : | 355818-98-3 |
MDL No. : | MFCD07778591 |
| Formula : |
C13H17N3O4
|
Boiling Point : |
- |
| Linear Structure Formula : | - |
InChI Key : | PBUXRZQTDKBQBM-UHFFFAOYSA-N |
| M.W : |
279.29
|
Pubchem ID : | 10934795 |
| Synonyms : |
|
Safety of [ 355818-98-3 ]
Application In Synthesis of [ 355818-98-3 ]
* All experimental methods are cited from the reference, please refer to the original source for details. We do not guarantee the accuracy of the content in the reference.
- Downstream synthetic route of [ 355818-98-3 ]
- 1
-
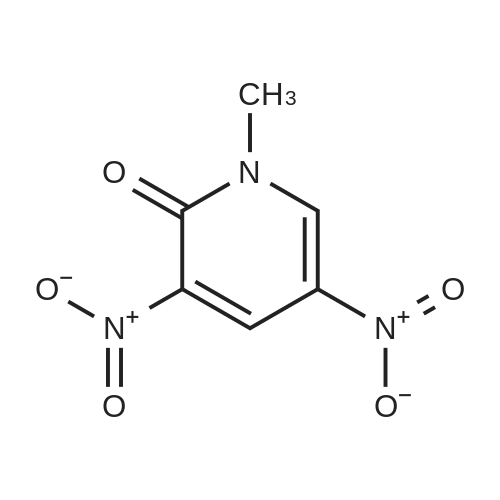 [ 14150-94-8 ]
[ 14150-94-8 ]

-
 [ 79099-07-3 ]
[ 79099-07-3 ]

-
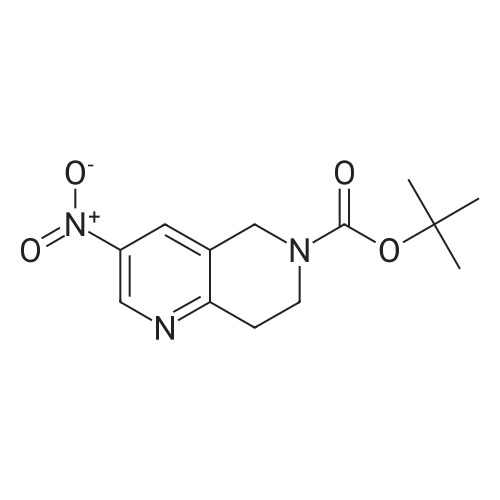 [ 355818-98-3 ]
[ 355818-98-3 ]
| Yield | Reaction Conditions | Operation in experiment |
| 67.76% |
With ammonia; In methanol; at 120℃; for 1h;Sealed tube; |
Step A: Ammonia gas (1.37 g, 80.32 mmol, 8 eq.) was added to a solution of 1-methyl-3,5-dinitro-pyridin-2-one (2g, 10.04 mmol, 1 eq.) and t-butyl 4-piperidone-1-carboxylate in methanol (50 mL) at room temperature. The reactionsolution was stirred in a sealed tank at 120C for 1 hour. The reaction solution was cooled down to room temperatureand concentrated to give a crude product. The crude product was purified by a silica gel chromatography column(PE/EA = 10/1 to 3/1) to give t-butyl 3-nitro-7,8-dihydro-5H-1,6-naphthyridine-6-carboxylate (1.9 g, 6.8 mmol, 67.76%yield) as a gray-white solid. |
| 67% |
With ammonia; In methanol; at 80℃; for 0.5h;Microwave irradiation; |
Into a 20 mL vial was placed <strong>[14150-94-8]1-methyl-3,5-dinitro-1,2-dihydropyridin-2-one</strong> (600 mg, 3.01 mmol), methanolic ammonia (10 mL), and tert-butyl 4-oxopiperidine-1-carboxylate (600 mg, 3.01 mmol). The reaction mixture was irradiated with microwave radiation for 30 min at 80 C then was concentrated under vacuum. The resulting residue was purified by column chromatography eluting with ethyl acetate/petroleum ether (1 : 1). The collected fractions were combined and concentrated under vacuum to yield tert-butyl 3-nitro-5,6,7,8-tetrahydro-1,6-naphthyridine-6-carboxylate (560 mg, 67%). |
|
With ammonia; In methanol; at 70℃; for 24h; |
2M solution of NH3 in MeOH (225 mL, 452.25 mmol) wasadded to a reaction vessel containing l-methyl-3,5-dinitro-lH-pyridin-2-one (6 g, 30.15 mmol) and 4-oxo-piperidine-l-carboxylic acid tert-butyl ester (6.6 g, 33.15 mmol). Thevessel was then sealed and the reaction was stirred for 24 hat 70 SC. After the resulting mixture was cooled to RT, thesolvent was removed to give crude product as yellow solid.After recrystallization in MeOH, the desired title compoundwas obtained as tan solid. MS (ES+) : 280.1 (M+H) + . Calc'dfor C13H17N304 - 279.12. |
|
With ammonia; In methanol; at 70℃; for 16h;sealed tube; |
l-Methyl-3,5-dinitro-lH-pyridin-2-one (6.0 g, 30.15 mmol), and 4-oxo-pirhoeridine-l- carboxylic acid tert-butyl ester (6.6 g, 33.15 mmol) were added to a 2 M solution OfNH3 in MeOH (225 mL, 452.3 mmol) in a sealed tube. The reaction was heated to 70 0C for 16 h. The solvent was removed and the yellow solid was recrystallized from MeOH affording 3-nitro-7,8-dihydro-5H-[l,6]naphthyridine-6-carboxylic acid tert-butyl ester as tan solid. |

Reference:
[1]Tetrahedron Letters,2008,vol. 49,p. 6368 - 6370
[2]Synthetic Communications,2001,vol. 31,p. 787 - 797
[3]Patent: EP3248968,2017,A1 .Location in patent: Paragraph 0092
[4]Patent: WO2019/200120,2019,A1 .Location in patent: Paragraph 000456
[5]Patent: WO2006/12374,2006,A1 .Location in patent: Page/Page column 228
[6]Bioorganic and Medicinal Chemistry Letters,2008,vol. 18,p. 4204 - 4209
[7]Patent: WO2007/48070,2007,A2 .Location in patent: Page/Page column 106
- 2
-
 [ 355818-98-3 ]
[ 355818-98-3 ]

-
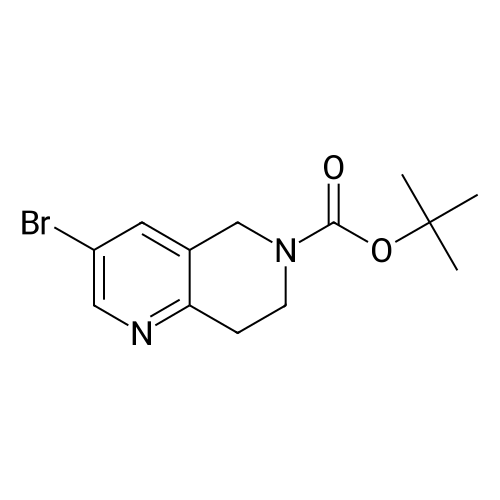 [ 1184950-48-8 ]
[ 1184950-48-8 ]

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science















 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping









