|
|
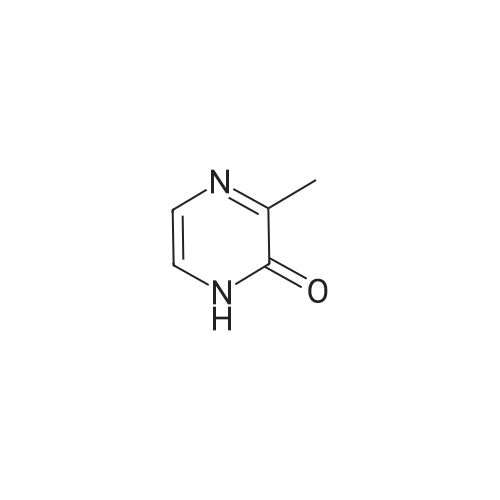
J is --NH(CH2 CH3) or is an amino acyl residue selected from the group consisting of D-alaninamide, N-(R0)-D-alaninamide, N-(R0)-L-alaninamide, alpha-aza-glycinamide, D-serinamide, and |
|
|
J is --NH(CH2 CH3) or is an amino acyl residue selected from the group consisting of D-alaninamide, alpha-aza-glycinamide, D-serinamide, and |
|
|
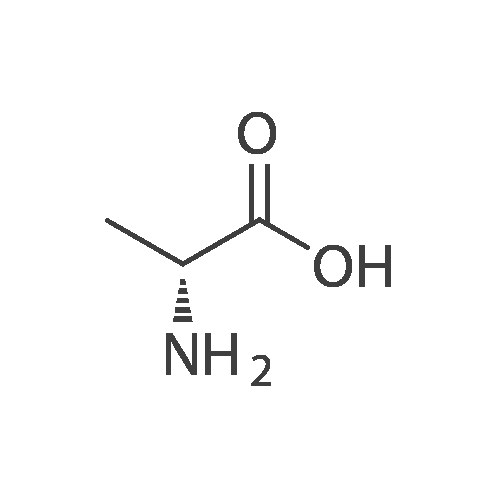
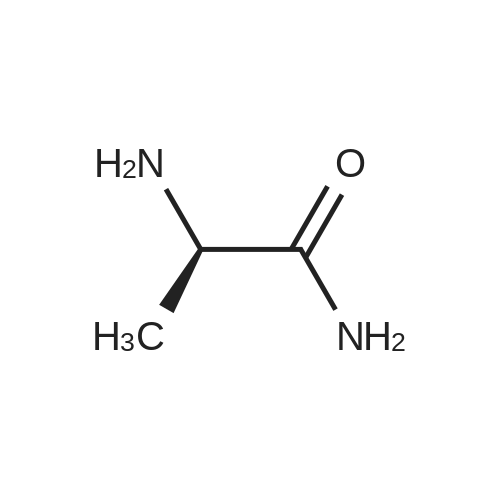
D. Coupling of D-alanine amide with L-aspartic acid N-thiocarboxyanhydride The D-alanine amide provided in Part C, 1.1 g (5.1 mmole) is dissolved in 5 ml of tetrahydrofuran and 5 ml of water is added. The clear solution is cooled in ice and 0.89 g (5.1 mmole) of L-aspartic acid N-thiocarboxyanhydride is added in one portion. To this is added as required, 0.5M sodium hydroxide to maintain the mixture at pH 9. After stirring 30 minutes the reaction mixture is washed with ethyl ether, then ethyl acetate and the washes are discarded. The aqueous phase is acidified with dilute hydrochloric acid to pH 5.6 and evaporated to dryness at reduced pressure. The residue is taken up in hot methanol, filtered and the methanol evaporated. The residue is taken up again in hot methanol, filtered and the filtrate decolorized with activated carbon, filtered through diatomaceous earth and the filtrate is evaporated to obtain the crude product. The crude product is dissolved in hot water and filtered, concentrated under a stream of nitrogen to a small volume and cooled to precipitate the title compound. Use of D-2-aminobutyric acid, D-serine, D-valine or D-2-aminopentanoic acid in place of D-alanine in the procedure of Part A above and reacting the N-t-butoxycarbonyl-D-amino carbonyl-D-amino acids thus obtained in the procedures of Parts B, C and D, similarly provides the corresponding compounds of formula (I) wherein R is 3,5-dimethyl-1,2-dithiolan-4-yl and Ra is C2 H5, CH2 OH, (CH3)2 CH, or CH3 CH2 CH2, respectively. |
|
|
Xaa11 is a hydroxy group or an amino acid amide selected from the group consisting of D-alanylamide, D-alanylethylamide, azaglycylamide, glycylamide, glycylethylamide, serylamide, D-serylamide, |
|
|
A compound according to claim 1 wherein Xaa11 is selected from the group consisting of D-alanylamide, D-alanylethylamide, azaglycylamide, NH-cyclobutyl, NH-cycloheptyl, NH-ethyl, glycylamide, glycylethylamide, NH-hexyl, ... |
|
|
Xaa11 is a hydroxy group or an amino acid amide selected from the group consisting of: alanylamide, D-alanylamide, alanylethylamide, D-alanylethylamide, azaglycylamide, glycylamide, glycylethylamide, N-methyl-D-alanylamide, serylamide, ... |
|
|
A compound according to claim 1, wherein Xaa11 is selected from the group consisting of alanylamide, D-alanylamide, alanylethylamide, D-alanylethylamide, azaglycylamide, NH-cyclobutyl, NH-cycloheptyl, NH-ethyl, NH-glycyl, ... |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science













 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping