| 70% |
With palladium 10% on activated carbon; hydrogen; In methanol; for 3.0h; |
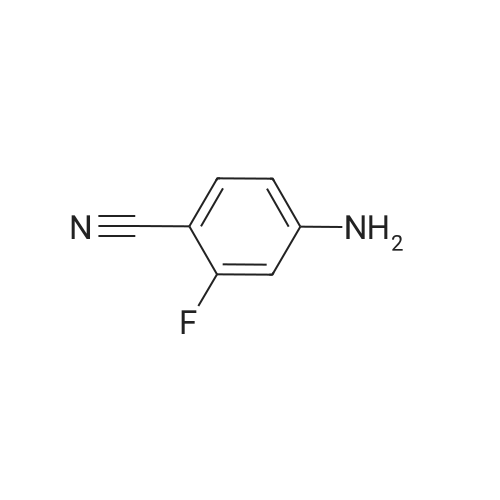
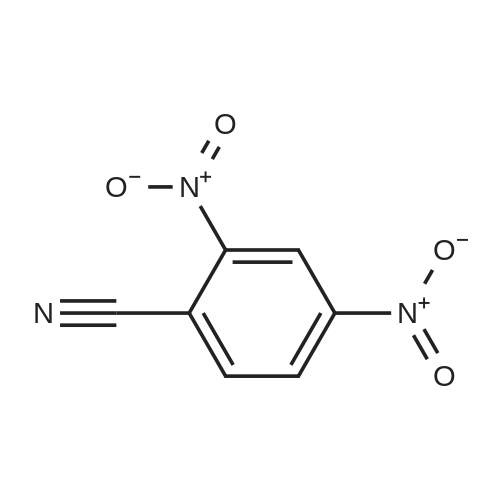
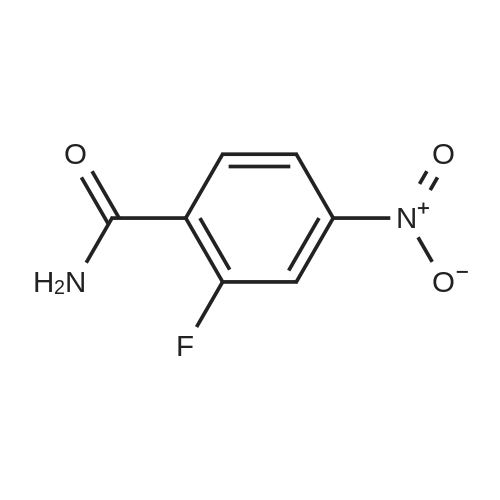
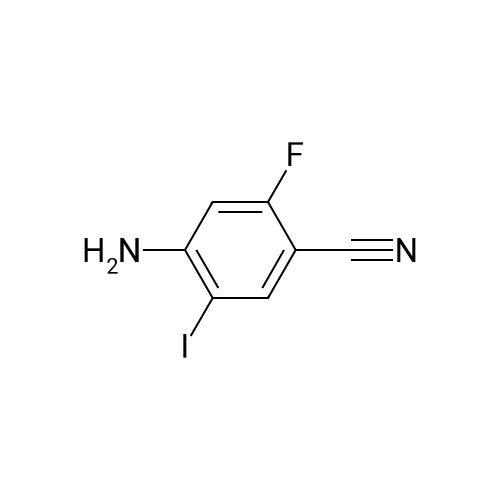
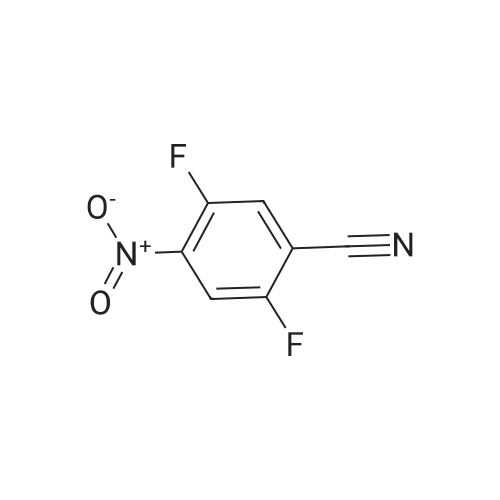
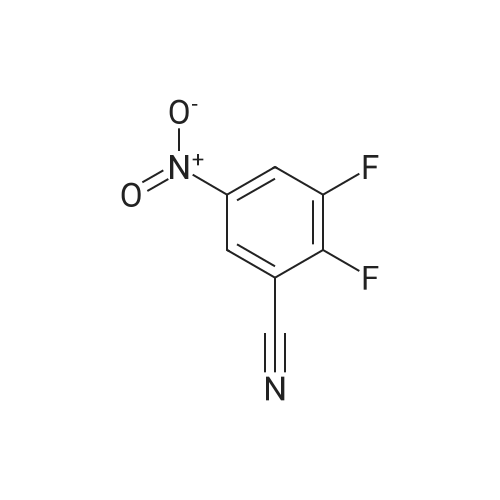
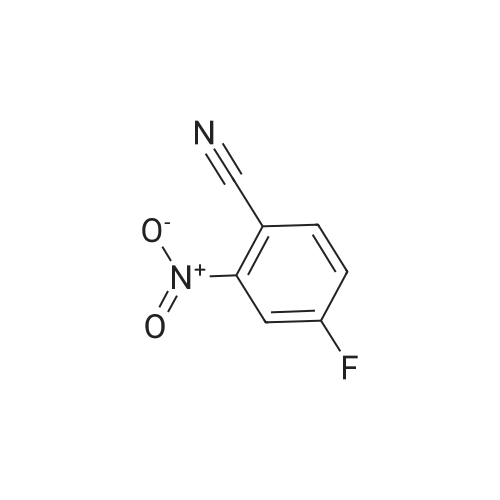
Reference Example 114 4-Amino-2-fluorobenzonitrile To a solution of <strong>[34667-88-4]2-fluoro-4-nitrobenzonitrile</strong> (2.51 g) in methanol (125 mL) was added 10percent palladium carbon (50percent containing water, 237 mg), and the mixture was stirred under a hydrogen atmosphere for 3 hr. The reaction mixture was filtrated, and the filtrate was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (eluent: hexane-ethyl acetate=1:1) to give the title compound as a pale-yellow solid (yield 1.43 g, 70percent). 1H-NMR (CDCl3)delta: 4.31 (2H, brs), 6.37-6.45 (2H, m), 7.31-7.36 (1H, m). |
|
palladium-carbon; In methanol; |
Reference Example 114 4-Amino-2-fluorobenzonitrile To a solution of <strong>[34667-88-4]2-fluoro-4-nitrobenzonitrile</strong> (2.51 g) in methanol (125 mL) was added 10percent palladium carbon (50percent containing water, 237 mg), and the mixture was stirred under a hydrogen atmosphere for 3 hr. The reaction mixture was filtrated, and the filtrate was concentrated under reduced pressure. The residue was purified by basic silica gel column chromatography (eluent: hexane-ethyl acetate=1:1) to give the title compound as a pale-yellow solid (yield 1.43 g, 70percent). 1H-NMR (CDCl3)delta: 4.31 (2H, brs), 6.37-6.45 (2H, m), 7.31-7.36 (1H, m). |
|
With hydrogen;5%-palladium/activated carbon; In methanol; at 20℃; under 1500.15 Torr; for 2.0h; |
A solution of <strong>[34667-88-4]2-fluoro-4-nitrobenzonitrile</strong> (5 g, 30 mmol) in 300 mL methanol is stirred in a hydrogenation reactor with palladium (5percent on activated charcoal, 400 mg) for 2 h at RT and 2 bar hydrogen pressure. The reaction mixture is filtered and evaporated down. Yield: 4 g. |
|
With iron; ammonium chloride; In ethanol; water; acetic acid; for 5.0h;Heating / reflux; |
Preparation 15: 4-Amino-2-fluorobenzonitrile: Saturated aqueous ammonium chloride (50 mL) and acetic acid (3 mL) were added to a stirred solution of <strong>[34667-88-4]2-fluoro-4-nitrobenzonitrile</strong> (50.0 g, 301 mmol) in EtOH (600 mL) followed by iron powder (2 g, 35.7 mmol). The mixture was heated under reflux and more iron powder (123 g, 2.20 mol) added portionwise over a 4 h period. The reaction heated for a further 1 h then allowed to cool to rt before being filtered through a celite plug. The filtrate was evaporated to dryness and the residual material partitioned between EtOAc (500 mL) and water (200 mL). The organic phase was washed with water (50 mL) and brine (100 mL), then dried (MgSO4) and evaporated to afford the title compound: deltaH (ddelta-DMSO) 6.41-6.46 (2H, m), 6.52 (2H, br s), 7.37- 7.41 (IH, m). |
|
With palladium 10% on activated carbon; hydrogen; In ethanol; acetonitrile; under 2585.81 Torr; for 1.0h; |
<strong>[34667-88-4]2-fluoro-4-nitrobenzonitrile</strong> (0.125 g, 0.751 mmol) was hydrogenated at 50 psi in the presence of 10percent Pd/C (40 mg) in EtOH/EtOAc (15mL) for 1 h. Filtered through Celite? and concentrated to an orange solid that was carried onto next step. MS (ESI) m/z: 137 (M+H)+. |
|
With palladium 10% on activated carbon; hydrogen; In methanol; at 20℃; for 12.0h; |
A solution of 66 6 (10.0g, 60.24mmol) in 69 methanol (100mL) was hydrogenated with 10percent 70 Pd/C (1.0g) under hydrogen atmosphere at room temperature for 12h. After filtration, the filtrate was evaporated to give the corresponding product. It was obtained as a gray 16 solid in 88percent yield. 7 was ready for the next step without the further purification. HRMS (ESI): m/z, calculated for C7H5FN2 137.0487 (M+H)+, found 137.0452. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 120K+ Compounds
120K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping